ZIP8-Mediated Intestinal Dysbiosis Impairs Pulmonary Host Defense against Bacterial Pneumonia
- PMID: 35162945
- PMCID: PMC8834709
- DOI: 10.3390/ijms23031022
ZIP8-Mediated Intestinal Dysbiosis Impairs Pulmonary Host Defense against Bacterial Pneumonia
Abstract
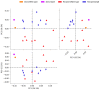
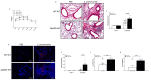
Pneumococcal pneumonia is a leading cause of morbidity and mortality worldwide. An increased susceptibility is due, in part, to compromised immune function. Zinc is required for proper immune function, and an insufficient dietary intake increases the risk of pneumonia. Our group was the first to reveal that the Zn transporter, ZIP8, is required for host defense. Furthermore, the gut microbiota that is essential for lung immunity is adversely impacted by a commonly occurring defective ZIP8 allele in humans. Taken together, we hypothesized that loss of the ZIP8 function would lead to intestinal dysbiosis and impaired host defense against pneumonia. To test this, we utilized a novel myeloid-specific Zip8KO mouse model in our studies. The comparison of the cecal microbial composition of wild-type and Zip8KO mice revealed significant differences in microbial community structure. Most strikingly, upon a S. pneumoniae lung infection, mice recolonized with Zip8KO-derived microbiota exhibited an increase in weight loss, bacterial dissemination, and lung inflammation compared to mice recolonized with WT microbiota. For the first time, we reveal the critical role of myeloid-specific ZIP8 on the maintenance of the gut microbiome structure, and that loss of ZIP8 leads to intestinal dysbiosis and impaired host defense in the lung. Given the high incidence of dietary Zn deficiency and the ZIP8 variant allele in the human population, additional investigation is warranted to improve surveillance and treatment strategies.
Keywords: gut-lung axis; host defense; microbiome; pneumonia; zinc; zinc transporter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- File T.M., Jr., Low D.E., Eckburg P.B., Talbot G.H., Friedland H.D., Lee J., Llorens L., Critchley I., Thye D. Integrated analysis of FOCUS 1 and FOCUS 2: Randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin. Infect. Dis. 2010;51:1395–1405. doi: 10.1086/657313. - DOI - PubMed
-
- Sherwin R.L., Gray S., Alexander R., McGovern P.C., Graepel J., Pride M.W., Purdy J., Paradiso P., File T.M., Jr. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J. Infect. Dis. 2013;208:1813–1820. doi: 10.1093/infdis/jit506. - DOI - PubMed
-
- Kaplan V., Angus D.C., Griffin M.F., Clermont G., Scott Watson R., Linde-Zwirble W.T. Hospitalized community-acquired pneumonia in the elderly: Age- and sex-related patterns of care and outcome in the United States. Am. J. Respir. Crit. Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

