Common T-Cell-Receptor Motifs and Features in Patients with Cytomegalovirus (CMV)-Seronegative End-Stage Renal Disease Receiving a Peptide Vaccination against CMV
- PMID: 35162953
- PMCID: PMC8835207
- DOI: 10.3390/ijms23031029
Common T-Cell-Receptor Motifs and Features in Patients with Cytomegalovirus (CMV)-Seronegative End-Stage Renal Disease Receiving a Peptide Vaccination against CMV
Abstract
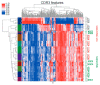
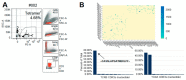
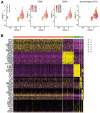
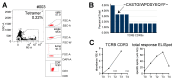
After solid-organ transplantation, reactivation of the cytomegalovirus (CMV) is often observed in seronegative patients and associated with a high risk of disease and mortality. CMV-specific T cells can prevent CMV reactivation. In a phase 1 trial, CMV-seronegative patients with end-stage renal disease listed for kidney transplantation were subjected to CMV phosphoprotein 65 (CMVpp65) peptide vaccination and further investigated for T-cell responses. To this end, CMV-specific CD8+ T cells were characterized by bulk T-cell-receptor (TCR) repertoire sequencing and combined single-cell RNA and TCR sequencing. In patients mounting an immune response to the vaccine, a common SYE(N)E TCR motif known to bind CMVpp65 was detected. CMV-peptide-vaccination-responder patients had TCR features distinct from those of non-responders. In a non-responder patient, a monoclonal inflammatory T-cell response was detected upon CMV reactivation. The identification of vaccine-induced CMV-reactive TCRs motifs might facilitate the development of cellular therapies for patients wait-listed for kidney transplantation.
Keywords: CMV; TCR motif; end-stage renal disease; peptide vaccination; single-cell sequencing.
Conflict of interest statement
The following authors of this manuscript have conflicts of interest to disclose: Apogenix: Funding for collaborative research. Hexal: Financial support for research on biosimilars and travel grants. Kite: Financial support for educational activities and conference and travel grants. A Co-PI for clinical trials on CAR-T cells. MSD: Ad board member, and a PI of clinical trials on letermovir. Novartis: Collaborative research grant for CAR-T cells. A Co-PI for clinical trials on CAR-T cells. TolerogenixX: Co-founder and shareholder. Conflict of interest (COI) declaration for AS: TolerogenixX: Co-founder and shareholder. Conflict of interest (COI) declaration for CS: Chiesi: Grant for research on biomarkers independent of the present study provided to the institution. Novartis: PI for clinical trials on immunosuppression. Calliditas Therapeutics: PI for a clinical trial on renal disease. All the other authors state no conflict of interest.
Figures


References
-
- Hodson E.M., A Jones C., Webster A.C., Strippoli G.F., Barclay P.G., Kable K., Vimalachandra D., Craig J.C. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: A systematic review of randomised controlled trials. Lancet. 2005;365:2105–2115. doi: 10.1016/S0140-6736(05)66553-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- BWST_ISF2018-046/Baden-Württemberg Stiftung (Michael Platten)
- SFB 1389-B03/Else Kröner-Fresenius Foundation (EKMS) and Deutsche Forschungsgemeinschaft, (Lukas Bunse)
- SFB 1389-B03/The CMVPepVac Study Scientific Committee designed the study, reviewed the study data and statistical analysis, and wrote the report. All authors had full access to the study data, decided to submit the report for publication, assume responsibility for the
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

