Emergent Roles of Circular RNAs in Metabolism and Metabolic Disorders
- PMID: 35162956
- PMCID: PMC8834750
- DOI: 10.3390/ijms23031032
Emergent Roles of Circular RNAs in Metabolism and Metabolic Disorders
Abstract
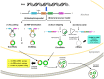
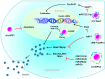
Circular RNAs (circRNAs) are an emerging group of long non-coding RNAs (lncRNAs) and have attracted attention again according to the progress in high-throughput sequencing in recent years. circRNAs are genome transcripts produced from pre-messenger (m)RNA regions in a specific process called "back-splicing," which forms covalently closed continuous loops. Due to their lack of a 5' cap and 3' poly-adenylated tails, circRNAs are remarkably more stable than linear RNAs. Functionally, circRNAs can endogenously sponge to microRNAs, interact with RNA-binding proteins (RBPs), or translate themselves. Moreover, circRNAs can be expressed in cell type- or tissue-specific expression patterns. Therefore, they are proposed to play essential roles in fine-tuning our body's homeostasis by regulating transcription and translation processes. Indeed, there has been accumulating emergent evidence showing that dysregulation of circRNAs can lead to metabolic disorders. This study explored the current knowledge of circRNAs that regulate molecular processes associated with glucose and lipid homeostasis and related pathogeneses of metabolic disorders. We also suggest the potential role of circRNAs as disease biomarkers and therapeutic targets.
Keywords: NAFLD; NASH; circRNA; diabetes; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Emerging Role and Promise of Circular RNAs in Obesity and Related Metabolic Disorders.Cells. 2020 Jun 16;9(6):1473. doi: 10.3390/cells9061473. Cells. 2020. PMID: 32560220 Free PMC article. Review.
-
Circular RNAs in physiology and non-immunological diseases.Trends Biochem Sci. 2022 Mar;47(3):250-264. doi: 10.1016/j.tibs.2021.11.004. Epub 2021 Dec 2. Trends Biochem Sci. 2022. PMID: 34865956 Review.
-
Role of Circular RNAs in Cardiovascular Disease.J Cardiovasc Pharmacol. 2020 Aug;76(2):128-137. doi: 10.1097/FJC.0000000000000841. J Cardiovasc Pharmacol. 2020. PMID: 32398477 Free PMC article. Review.
-
Circular RNA-protein interactions: functions, mechanisms, and identification.Theranostics. 2020 Feb 10;10(8):3503-3517. doi: 10.7150/thno.42174. eCollection 2020. Theranostics. 2020. PMID: 32206104 Free PMC article. Review.
-
The roles of circRNAs in cancers: Perspectives from molecular functions.Gene. 2021 Jan 30;767:145182. doi: 10.1016/j.gene.2020.145182. Epub 2020 Sep 28. Gene. 2021. PMID: 32991954 Review.
Cited by
-
Post-transcriptional control by RNA-binding proteins in diabetes and its related complications.Front Physiol. 2022 Oct 6;13:953880. doi: 10.3389/fphys.2022.953880. eCollection 2022. Front Physiol. 2022. PMID: 36277184 Free PMC article. Review.
-
Effects of circulating RNAs on tumor metabolism in lung cancer (Review).Oncol Lett. 2025 Feb 27;29(4):204. doi: 10.3892/ol.2025.14950. eCollection 2025 Apr. Oncol Lett. 2025. PMID: 40070786 Free PMC article. Review.
-
Circular RNA, A Molecule with Potential Chemistry and Applications in RNA-based Cancer Therapeutics: An Insight into Recent Advances.Top Curr Chem (Cham). 2025 May 9;383(2):21. doi: 10.1007/s41061-025-00505-z. Top Curr Chem (Cham). 2025. PMID: 40343623 Free PMC article. Review.
-
Profiling of differentially expressed MicroRNAs in familial hypercholesterolemia via direct hybridization.Noncoding RNA Res. 2024 Mar 8;9(3):796-810. doi: 10.1016/j.ncrna.2024.02.017. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38590435 Free PMC article.
-
APOB CRISPR-Cas9 Engineering in Hypobetalipoproteinemia: A Promising Tool for Functional Studies of Novel Variants.Int J Mol Sci. 2022 Apr 13;23(8):4281. doi: 10.3390/ijms23084281. Int J Mol Sci. 2022. PMID: 35457099 Free PMC article.
References
-
- Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. [(accessed on 3 December 2020)]. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-est....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

