Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection
- PMID: 35162972
- PMCID: PMC8835123
- DOI: 10.3390/ijms23031049
Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection
Abstract
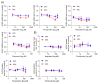
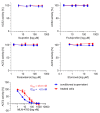
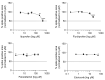
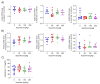
SARS-CoV-2 uses the human cell surface protein angiotensin converting enzyme 2 (ACE2) as the receptor by which it gains access into lung and other tissue. Early in the pandemic, there was speculation that a number of commonly used medications-including ibuprofen and other non-steroidal anti-inflammatory drugs (NSAIDs)-have the potential to upregulate ACE2, thereby possibly facilitating viral entry and increasing the severity of COVID-19. We investigated the influence of the NSAIDS with a range of cyclooxygenase (COX)1 and COX2 selectivity (ibuprofen, flurbiprofen, etoricoxib) and paracetamol on the level of ACE2 mRNA/protein expression and activity as well as their influence on SARS-CoV-2 infection levels in a Caco-2 cell model. We also analysed the ACE2 mRNA/protein levels and activity in lung, heart and aorta in ibuprofen treated mice. The drugs had no effect on ACE2 mRNA/protein expression and activity in the Caco-2 cell model. There was no up-regulation of ACE2 mRNA/protein expression and activity in lung, heart and aorta tissue in ibuprofen-treated mice in comparison to untreated mice. Viral load was significantly reduced by both flurbiprofen and ibuprofen at high concentrations. Ibuprofen, flurbiprofen, etoricoxib and paracetamol demonstrated no effects on ACE2 expression or activity in vitro or in vivo. Higher concentrations of ibuprofen and flurbiprofen reduced SARS-CoV-2 replication in vitro.
Keywords: ACE protein expression; ACE2 activity; ACE2 mRNA expression; NSAIDs; SARS-CoV-2 infection; ibuprofen.
Conflict of interest statement
The authors P.R., A.S., D.B., S.T., J.C., S.C., N.d.B., P.G., A.K., S.S. (Susanne Schiffmann), declare no competing interests. The authors B.C., S.S. (Simon Sinclair), I.L., G.P., W.F.L. are employees of Reckitt, the owners and distributors of the Nurofen brand.
Figures
References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. - DOI - PMC - PubMed
-
- Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., Yee N.T.S., Liu C., Nerurkar S.N., Kai J.C.Y., et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

