GILT Expression in Human Melanoma Cells Enhances Generation of Antigenic Peptides for HLA Class II-Mediated Immune Recognition
- PMID: 35162988
- PMCID: PMC8835040
- DOI: 10.3390/ijms23031066
GILT Expression in Human Melanoma Cells Enhances Generation of Antigenic Peptides for HLA Class II-Mediated Immune Recognition
Abstract
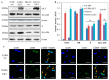
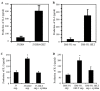
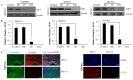
Melanoma is an aggressive skin cancer that has become increasingly prevalent in western populations. Current treatments such as surgery, chemotherapy, and high-dose radiation have had limited success, often failing to treat late stage, metastatic melanoma. Alternative strategies such as immunotherapies have been successful in treating a small percentage of patients with metastatic disease, although these treatments to date have not been proven to enhance overall survival. Several melanoma antigens (Ags) proposed as targets for immunotherapeutics include tyrosinase, NY-ESO-1, gp-100, and Mart-1, all of which contain both human leukocyte antigen (HLA) class I and class II-restricted epitopes necessary for immune recognition. We have previously shown that an enzyme, gamma-IFN-inducible lysosomal thiol-reductase (GILT), is abundantly expressed in professional Ag presenting cells (APCs), but absent or expressed at greatly reduced levels in many human melanomas. In the current study, we report that increased GILT expression generates a greater pool of antigenic peptides in melanoma cells for enhanced CD4+ T cell recognition. Our results suggest that the induction of GILT in human melanoma cells could aid in the development of a novel whole-cell vaccine for the enhancement of immune recognition of metastatic melanoma.
Keywords: Ag presenting cells (APCs); CD4+ T cells; cathepsins; gamma-IFN-inducible lysosomal thiol-reductase (GILT); human leukocyte antigen (HLA) class II; melanoma.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures






Similar articles
-
Gamma-IFN-inducible-lysosomal thiol reductase modulates acidic proteases and HLA class II antigen processing in melanoma.Cancer Immunol Immunother. 2008 Oct;57(10):1461-70. doi: 10.1007/s00262-008-0483-8. Epub 2008 Mar 15. Cancer Immunol Immunother. 2008. PMID: 18343923 Free PMC article.
-
Gamma-interferon-inducible lysosomal thiol reductase is upregulated in human melanoma.Melanoma Res. 2016 Apr;26(2):125-37. doi: 10.1097/CMR.0000000000000230. Melanoma Res. 2016. PMID: 26930048 Free PMC article.
-
GILT in Thymic Epithelial Cells Facilitates Central CD4 T Cell Tolerance to a Tissue-Restricted, Melanoma-Associated Self-Antigen.J Immunol. 2020 Jun 1;204(11):2877-2886. doi: 10.4049/jimmunol.1900523. Epub 2020 Apr 8. J Immunol. 2020. PMID: 32269095 Free PMC article.
-
Diverse cellular and organismal functions of the lysosomal thiol reductase GILT.Mol Immunol. 2015 Dec;68(2 Pt A):124-8. doi: 10.1016/j.molimm.2015.06.008. Epub 2015 Jun 23. Mol Immunol. 2015. PMID: 26116226 Free PMC article. Review.
-
Disulfide reduction in the endocytic pathway: immunological functions of gamma-interferon-inducible lysosomal thiol reductase.Antioxid Redox Signal. 2011 Aug 1;15(3):657-68. doi: 10.1089/ars.2010.3684. Epub 2011 Apr 20. Antioxid Redox Signal. 2011. PMID: 21506690 Free PMC article. Review.
Cited by
-
Inflammation in Health and Disease: New Insights and Therapeutic Avenues.Int J Mol Sci. 2022 Jul 29;23(15):8392. doi: 10.3390/ijms23158392. Int J Mol Sci. 2022. PMID: 35955527 Free PMC article.
-
The multifaceted roles of cathepsins in immune and inflammatory responses: implications for cancer therapy, autoimmune diseases, and infectious diseases.Biomark Res. 2024 Dec 31;12(1):165. doi: 10.1186/s40364-024-00711-9. Biomark Res. 2024. PMID: 39736788 Free PMC article. Review.
-
Peptide Modification Diminishes HLA Class II-restricted CD4+ T Cell Recognition of Prostate Cancer Cells.Int J Mol Sci. 2022 Dec 3;23(23):15234. doi: 10.3390/ijms232315234. Int J Mol Sci. 2022. PMID: 36499557 Free PMC article.
-
Extracellular RNA in melanoma: Advances, challenges, and opportunities.Front Cell Dev Biol. 2023 May 4;11:1141543. doi: 10.3389/fcell.2023.1141543. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37215082 Free PMC article. Review.
-
High GILT Expression Is Associated with Improved Survival in Metastatic Melanoma Patients Treated with Immune Checkpoint Inhibition.Cancers (Basel). 2022 Apr 28;14(9):2200. doi: 10.3390/cancers14092200. Cancers (Basel). 2022. PMID: 35565329 Free PMC article.
References
-
- van der Kooij M.K., Dekkers O.M., Aarts M.J.B., van den Berkmortel F.W.P.J., Boers-Sonderen M.J., de Groot J.W.B., Hospers G.A.P., Piersma D., van Rijn R.S., Suijkerbuijk K.P.M., et al. Sex-Based Differences in Treatment with Immune Checkpoint Inhibition and Targeted Therapy for Advanced Melanoma: A Nationwide Cohort Study. Cancers. 2021;13:4639. doi: 10.3390/cancers13184639. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

