The Effect of TGF-β1 Reduced Functionality on the Expression of Selected Synaptic Proteins and Electrophysiological Parameters: Implications of Changes Observed in Acute Hepatic Encephalopathy
- PMID: 35163004
- PMCID: PMC8835518
- DOI: 10.3390/ijms23031081
The Effect of TGF-β1 Reduced Functionality on the Expression of Selected Synaptic Proteins and Electrophysiological Parameters: Implications of Changes Observed in Acute Hepatic Encephalopathy
Abstract
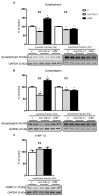
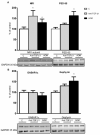
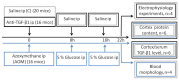
Decreased platelet count represents a feature of acute liver failure (ALF) pathogenesis. Platelets are the reservoir of transforming growth factor 1 (TGF-β1), a multipotent cytokine involved in the maintenance of, i.a., central nervous system homeostasis. Here, we analyzed the effect of a decrease in TGF-β1 active form on synaptic proteins levels, and brain electrophysiology, in mice after intraperitoneal (ip) administration of TGF-β1 antibody (anti-TGF-β1; 1 mg/mL). Next, we correlated it with a thrombocytopenia-induced TGF-β1 decrease, documented in an azoxymethane-induced (AOM; 100 mM ip) model of ALF, and clarified the impact of TGF-β1 decrease on blood-brain barrier functionality. The increase of both synaptophysin and synaptotagmin in the cytosolic fraction, and its reduction in a membrane fraction, were confirmed in the AOM mice brains. Both proteins' decrease in analyzed fractions occurred in anti-TGF-β1 mice. In turn, an increase in postsynaptic (NR1 subunit of N-methyl-D-aspartate receptor, postsynaptic density protein 95, gephyrin) proteins in the AOM brain cortex, but a selective compensatory increase of NR1 subunit in anti-TGF-β mice, was observed. The alterations of synaptic proteins levels were not translated on electrophysiological parameters in the anti-TGF-β1 model. The results suggest the impairment of synaptic vesicles docking to the postsynaptic membrane in the AOM model. Nevertheless, changes in synaptic protein level in the anti-TGF-β1 mice do not affect neurotransmission and may not contribute to neurologic deficits in AOM mice.
Keywords: LTP; acute liver failure; blood–brain barrier; glutamatergic transmission; synaptic proteins; transforming growth factor β1.
Conflict of interest statement
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest concerning this manuscript.
Figures



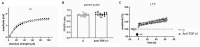
Similar articles
-
Thrombospondin-1 Exacerbates Acute Liver Failure and Hepatic Encephalopathy Pathology in Mice by Activating Transforming Growth Factor β1.Am J Pathol. 2020 Feb;190(2):347-357. doi: 10.1016/j.ajpath.2019.10.003. Epub 2019 Nov 14. Am J Pathol. 2020. PMID: 31734229 Free PMC article.
-
Elevated circulating TGFβ1 during acute liver failure activates TGFβR2 on cortical neurons and exacerbates neuroinflammation and hepatic encephalopathy in mice.J Neuroinflammation. 2019 Apr 2;16(1):69. doi: 10.1186/s12974-019-1455-y. J Neuroinflammation. 2019. PMID: 30940161 Free PMC article.
-
TGFβ1 exacerbates blood-brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5.Lab Invest. 2015 Aug;95(8):903-13. doi: 10.1038/labinvest.2015.70. Epub 2015 Jun 1. Lab Invest. 2015. PMID: 26006017 Free PMC article.
-
Cerebral blood flow in acute liver failure: a finding in search of a mechanism.Metab Brain Dis. 2004 Dec;19(3-4):177-94. doi: 10.1023/b:mebr.0000043968.04313.e7. Metab Brain Dis. 2004. PMID: 15554414 Review.
-
Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema.Metab Brain Dis. 2013 Jun;28(2):145-50. doi: 10.1007/s11011-012-9361-3. Epub 2012 Dec 5. Metab Brain Dis. 2013. PMID: 23212479 Review.
Cited by
-
Cardiomyocyte-fibroblast crosstalk in the postnatal heart.Front Cell Dev Biol. 2023 Apr 3;11:1163331. doi: 10.3389/fcell.2023.1163331. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37077417 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

