Reduction in Ventilation-Induced Diaphragmatic Mitochondrial Injury through Hypoxia-Inducible Factor 1α in a Murine Endotoxemia Model
- PMID: 35163007
- PMCID: PMC8835058
- DOI: 10.3390/ijms23031083
Reduction in Ventilation-Induced Diaphragmatic Mitochondrial Injury through Hypoxia-Inducible Factor 1α in a Murine Endotoxemia Model
Abstract
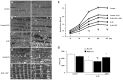
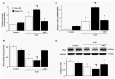
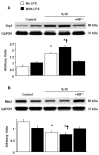
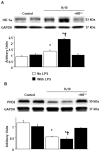
Mechanical ventilation (MV) is essential for patients with sepsis-related respiratory failure but can cause ventilator-induced diaphragm dysfunction (VIDD), which involves diaphragmatic myofiber atrophy and contractile inactivity. Mitochondrial DNA, oxidative stress, mitochondrial dynamics, and biogenesis are associated with VIDD. Hypoxia-inducible factor 1α (HIF-1α) is crucial in the modulation of diaphragm immune responses. The mechanism through which HIF-1α and mitochondria affect sepsis-related diaphragm injury is unknown. We hypothesized that MV with or without endotoxin administration would aggravate diaphragmatic and mitochondrial injuries through HIF-1α. C57BL/6 mice, either wild-type or HIF-1α-deficient, were exposed to MV with or without endotoxemia for 8 h. MV with endotoxemia augmented VIDD and mitochondrial damage, which presented as increased oxidative loads, dynamin-related protein 1 level, mitochondrial DNA level, and the expressions of HIF-1α and light chain 3-II. Furthermore, disarrayed myofibrils; disorganized mitochondria; increased autophagosome numbers; and substantially decreased diaphragm contractility, electron transport chain activities, mitofusin 2, mitochondrial transcription factor A, peroxisome proliferator activated receptor-γ coactivator-1α, and prolyl hydroxylase domain 2 were observed (p < 0.05). Endotoxin-stimulated VIDD and mitochondrial injuries were alleviated in HIF-1α-deficient mice (p < 0.05). Our data revealed that endotoxin aggravated MV-induced diaphragmatic dysfunction and mitochondrial damages, partially through the HIF-1α signaling pathway.
Keywords: autophagy; biogenesis; dynamics; hypoxia-inducible factor-1α; mitochondria; ventilator-induced diaphragm dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Horn A.G., Davis R.T., III., Baumfalk D.R., Kunkel O.N., Bruells C.S., McCullough D.J., Opoku-Acheampong A.B., Poole D.C., Behnke B.J. Impaired diaphragm resistance vessel vasodilation with prolonged mechanical ventilation. J. Appl. Physiol. 2019;127:423–431. doi: 10.1152/japplphysiol.00189.2019. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

