Conductive Polymer PEDOT:PSS-Based Platform for Embryonic Stem-Cell Differentiation
- PMID: 35163031
- PMCID: PMC8835127
- DOI: 10.3390/ijms23031107
Conductive Polymer PEDOT:PSS-Based Platform for Embryonic Stem-Cell Differentiation
Abstract
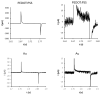
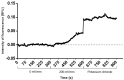
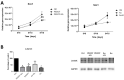
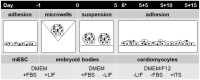
Organic semiconductors are constantly gaining interest in regenerative medicine. Their tunable physico-chemical properties, including electrical conductivity, are very promising for the control of stem-cell differentiation. However, their use for combined material-based and electrical stimulation remains largely underexplored. Therefore, we carried out a study on whether a platform based on the conductive polymer poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) can be beneficial to the differentiation of mouse embryonic stem cells (mESCs). The platform was prepared using the layout of a standard 24-well cell-culture plate. Polyethylene naphthalate foil served as the substrate for the preparation of interdigitated gold electrodes by physical vapor deposition. The PEDOT:PSS pattern was fabricated by precise screen printing over the gold electrodes. The PEDOT:PSS platform was able to produce higher electrical current with the pulsed-direct-current (DC) electrostimulation mode (1 Hz, 200 mV/mm, 100 ms pulse duration) compared to plain gold electrodes. There was a dominant capacitive component. In proof-of-concept experiments, mESCs were able to respond to such electrostimulation by membrane depolarization and elevation of cytosolic calcium. Further, the PEDOT:PSS platform was able to upregulate cardiomyogenesis and potentially inhibit early neurogenesis per se with minor contribution of electrostimulation. Hence, the present work highlights the large potential of PEDOT:PSS in regenerative medicine.
Keywords: PEDOT:PSS; conductive polymer; electrostimulation; embryonic stem cells; screen print.
Conflict of interest statement
The authors declare no conflict of interest. The funding bodies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:582-590. doi: 10.1016/j.msec.2019.02.008. Epub 2019 Feb 2. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889733
-
Highly Conductive PEDOT:PSS Films with 1,3-Dimethyl-2-Imidazolidinone as Transparent Electrodes for Organic Light-Emitting Diodes.Macromol Rapid Commun. 2016 Sep;37(17):1427-33. doi: 10.1002/marc.201600144. Epub 2016 Jul 5. Macromol Rapid Commun. 2016. PMID: 27377555
-
An Inkjet-Printed PEDOT:PSS-Based Stretchable Conductor for Wearable Health Monitoring Device Applications.ACS Appl Mater Interfaces. 2021 May 12;13(18):21693-21702. doi: 10.1021/acsami.1c00537. Epub 2021 Apr 29. ACS Appl Mater Interfaces. 2021. PMID: 33926183
-
Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS.Adv Mater. 2019 Mar;31(10):e1806133. doi: 10.1002/adma.201806133. Epub 2019 Jan 2. Adv Mater. 2019. PMID: 30600559 Free PMC article. Review.
-
Advancements in tailoring PEDOT: PSS properties for bioelectronic applications: A comprehensive review.Biomater Adv. 2023 Nov;154:213655. doi: 10.1016/j.bioadv.2023.213655. Epub 2023 Oct 10. Biomater Adv. 2023. PMID: 37866232 Review.
Cited by
-
Transcriptomic Analysis Reveals the Heterogeneous Role of Conducting Films Upon Electrical Stimulation.Adv Healthc Mater. 2024 Dec;13(32):e2400364. doi: 10.1002/adhm.202400364. Epub 2024 Sep 2. Adv Healthc Mater. 2024. PMID: 39221662 Free PMC article.
-
Synergy of Nanotopography and Electrical Conductivity of PEDOT/PSS for Enhanced Neuronal Development.ACS Appl Mater Interfaces. 2023 Dec 27;15(51):59224-59235. doi: 10.1021/acsami.3c15278. Epub 2023 Dec 13. ACS Appl Mater Interfaces. 2023. PMID: 38091494 Free PMC article.
-
Global, regional, and national epidemiology of congenital heart disease in children from 1990 to 2021.Front Cardiovasc Med. 2025 May 16;12:1522644. doi: 10.3389/fcvm.2025.1522644. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40454242 Free PMC article.
-
PEDOT: PSS promotes neurogenic commitment of neural crest-derived stem cells.Front Physiol. 2022 Aug 17;13:930804. doi: 10.3389/fphys.2022.930804. eCollection 2022. Front Physiol. 2022. PMID: 36060701 Free PMC article.
-
Electroactive 4D Porous Scaffold Based on Conducting Polymer as a Responsive and Dynamic In Vitro Cell Culture Platform.ACS Appl Mater Interfaces. 2024 Feb 7;16(5):5613-5626. doi: 10.1021/acsami.3c16686. Epub 2024 Jan 26. ACS Appl Mater Interfaces. 2024. PMID: 38278772 Free PMC article.
References
-
- Tian H.-C., Liu J.-Q., Kang X.-Y., Wei D.-X., Zhang C., Du J.-C., Yang B., Chen X., Yang C.-S. Biotic and abiotic molecule dopants determining the electrochemical performance, stability and fibroblast behavior of conducting polymer for tissue interface. RSC Adv. 2014;4:47461–47471. doi: 10.1039/C4RA07265K. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

