Cell-Cell Mating Interactions: Overview and Potential of Single-Cell Force Spectroscopy
- PMID: 35163034
- PMCID: PMC8835621
- DOI: 10.3390/ijms23031110
Cell-Cell Mating Interactions: Overview and Potential of Single-Cell Force Spectroscopy
Abstract
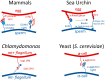
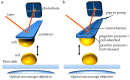
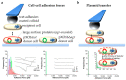
It is an understatement that mating and DNA transfer are key events for living organisms. Among the traits needed to facilitate mating, cell adhesion between gametes is a universal requirement. Thus, there should be specific properties for the adhesion proteins involved in mating. Biochemical and biophysical studies have revealed structural information about mating adhesins, as well as their specificities and affinities, leading to some ideas about these specialized adhesion proteins. Recently, single-cell force spectroscopy (SCFS) has added important findings. In SCFS, mating cells are brought into contact in an atomic force microscope (AFM), and the adhesive forces are monitored through the course of mating. The results have shown some remarkable characteristics of mating adhesins and add knowledge about the design and evolution of mating adhesins.
Keywords: adhesion; atomic force microscopy; bacteria; cell–cell mating; conjugation; gametes; single-cell force spectroscopy; yeasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Measuring Mechanical and Adhesive Properties of Single Cells Using an Atomic Force Microscope.Methods Mol Biol. 2021;2294:81-92. doi: 10.1007/978-1-0716-1350-4_6. Methods Mol Biol. 2021. PMID: 33742395
-
Examining Cell-Cell Interactions in the Kidney Using AFM Single-Cell Force Spectroscopy.Methods Mol Biol. 2020;2067:189-201. doi: 10.1007/978-1-4939-9841-8_14. Methods Mol Biol. 2020. PMID: 31701454
-
Quantifying the forces guiding microbial cell adhesion using single-cell force spectroscopy.Nat Protoc. 2014 May;9(5):1049-55. doi: 10.1038/nprot.2014.066. Epub 2014 Apr 10. Nat Protoc. 2014. PMID: 24722404
-
Single-cell force spectroscopy, an emerging tool to quantify cell adhesion to biomaterials.Tissue Eng Part B Rev. 2014 Feb;20(1):40-55. doi: 10.1089/ten.TEB.2013.0125. Epub 2013 Jul 10. Tissue Eng Part B Rev. 2014. PMID: 23688177 Review.
-
A practical guide to quantify cell adhesion using single-cell force spectroscopy.Methods. 2013 Apr 1;60(2):169-78. doi: 10.1016/j.ymeth.2013.01.006. Epub 2013 Feb 8. Methods. 2013. PMID: 23396062 Review.
Cited by
-
A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies.Nanomaterials (Basel). 2023 Sep 18;13(18):2585. doi: 10.3390/nano13182585. Nanomaterials (Basel). 2023. PMID: 37764614 Free PMC article. Review.
-
AFM reveals differential effects of acidification on LDL- and oxidized LDL-receptor interactions: biomechanical implications in atherogenesis.Cell Mol Biol Lett. 2025 Mar 18;30(1):32. doi: 10.1186/s11658-025-00715-9. Cell Mol Biol Lett. 2025. PMID: 40102716 Free PMC article.
-
Interdomain Linker Effect on the Mechanical Stability of Ig Domains in Titin.Int J Mol Sci. 2022 Aug 30;23(17):9836. doi: 10.3390/ijms23179836. Int J Mol Sci. 2022. PMID: 36077234 Free PMC article.
-
Special Issue: Yeast Cell Signaling Pathways (Volume 1).Int J Mol Sci. 2023 Mar 3;24(5):4929. doi: 10.3390/ijms24054929. Int J Mol Sci. 2023. PMID: 36902357 Free PMC article.
-
Perceiving and Countering Marine Biofouling: Structure, Forces, and Processes at Surfaces in Sea Water Across the Length Scales.Langmuir. 2025 Apr 1;41(12):7996-8018. doi: 10.1021/acs.langmuir.5c00450. Epub 2025 Mar 20. Langmuir. 2025. PMID: 40113572 Free PMC article. Review.
References
-
- D’Occhio M.J., Campanile G., Zicarelli L., Visintin J.A., Baruselli P.S. Adhesion Molecules in Gamete Transport, Fertilization, Early Embryonic Development, and Implantation-Role in Establishing a Pregnancy in Cattle: A Review. Mol. Reprod. Dev. 2020;87:206–222. doi: 10.1002/mrd.23312. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

