Inflammation Regulates Haematopoietic Stem Cells and Their Niche
- PMID: 35163048
- PMCID: PMC8835214
- DOI: 10.3390/ijms23031125
Inflammation Regulates Haematopoietic Stem Cells and Their Niche
Abstract
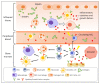
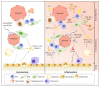
Haematopoietic stem cells (HSCs) reside in the bone marrow and are supported by the specialised microenvironment, a niche to maintain HSC quiescence. To deal with haematopoietic equilibrium disrupted during inflammation, HSCs are activated from quiescence directly and indirectly to generate more mature immune cells, especially the myeloid lineage cells. In the process of proliferation and differentiation, HSCs gradually lose their self-renewal potential. The extensive inflammation might cause HSC exhaustion/senescence and malignant transformation. Here, we summarise the current understanding of how HSC functions are maintained, damaged, or exhausted during acute, prolonged, and pathological inflammatory conditions. We also highlight the inflammation-altered HSC niche and its impact on escalating the insults on HSCs.
Keywords: bone marrow niche; haematopoietic stem cells; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inflammation and Aging of Hematopoietic Stem Cells in Their Niche.Cells. 2021 Jul 21;10(8):1849. doi: 10.3390/cells10081849. Cells. 2021. PMID: 34440618 Free PMC article. Review.
-
Regulation of hematopoietic stem cells in the niche.Sci China Life Sci. 2015 Dec;58(12):1209-15. doi: 10.1007/s11427-015-4960-y. Epub 2015 Nov 13. Sci China Life Sci. 2015. PMID: 26563156 Review.
-
Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1.Nat Commun. 2021 Jan 27;12(1):608. doi: 10.1038/s41467-020-20801-0. Nat Commun. 2021. PMID: 33504783 Free PMC article.
-
Regulation of hematopoietic and leukemia stem cells by regulatory T cells.Front Immunol. 2022 Nov 2;13:1049301. doi: 10.3389/fimmu.2022.1049301. eCollection 2022. Front Immunol. 2022. PMID: 36405718 Free PMC article. Review.
-
Niche regulation of hematopoietic stem cells in the endosteum.Ann N Y Acad Sci. 2009 Sep;1176:36-46. doi: 10.1111/j.1749-6632.2009.04561.x. Ann N Y Acad Sci. 2009. PMID: 19796231
Cited by
-
Role of Neurotransmitters in Steady State Hematopoiesis, Aging, and Leukemia.Stem Cell Rev Rep. 2025 Jan;21(1):2-27. doi: 10.1007/s12015-024-10761-z. Epub 2024 Jul 8. Stem Cell Rev Rep. 2025. PMID: 38976142 Review.
-
Hematopoietic stem cell metabolism within the bone marrow niche - insights and opportunities.Bioessays. 2025 Feb;47(2):e2400154. doi: 10.1002/bies.202400154. Epub 2024 Nov 6. Bioessays. 2025. PMID: 39506498 Free PMC article. Review.
-
Bone marrow-fibroblast progenitor cell-derived small extracellular vesicles promote cardiac fibrosis via miR-21-5p and integrin subunit αV signalling.J Extracell Biol. 2024 Jun 22;3(6):e152. doi: 10.1002/jex2.152. eCollection 2024 Jun. J Extracell Biol. 2024. PMID: 38947170 Free PMC article.
-
A Perfect Storm: The Convergence of Aging, Human Immunodeficiency Virus Infection, and Inflammasome Dysregulation.Curr Issues Mol Biol. 2024 May 15;46(5):4768-4786. doi: 10.3390/cimb46050287. Curr Issues Mol Biol. 2024. PMID: 38785555 Free PMC article. Review.
-
Immune aging and infectious diseases.Chin Med J (Engl). 2024 Dec 20;137(24):3010-3049. doi: 10.1097/CM9.0000000000003410. Epub 2024 Dec 16. Chin Med J (Engl). 2024. PMID: 39679477 Free PMC article. Review.
References
-
- Kakiuchi M., Hirata Y., Robson S.C., Fujisaki J. Paradoxical Regulation of Allogeneic Bone Marrow Engraftment and Immune Privilege by Mesenchymal Cells and Adenosine. Transplant. Cell. Ther. Off. Publ. Am. Soc. Transplant. Cell. Ther. 2021;27:92.e1–92.e5. doi: 10.1016/j.bbmt.2020.09.017. - DOI - PMC - PubMed
-
- Contreras-Lopez R., Elizondo-Vega R., Paredes M.J., Luque-Campos N., Torres M.J., Tejedor G., Vega-Letter A.M., Figueroa-Valdés A., Pradenas C., Oyarce K., et al. HIF1α-Dependent Metabolic Reprogramming Governs Mesenchymal Stem/Stromal Cell Immunoregulatory Functions. FASEB J. 2020;34:8250–8264. doi: 10.1096/fj.201902232R. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

