Rab21 Protein Is Degraded by Both the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway
- PMID: 35163051
- PMCID: PMC8835697
- DOI: 10.3390/ijms23031131
Rab21 Protein Is Degraded by Both the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway
Abstract
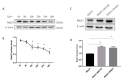
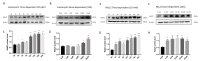
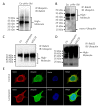
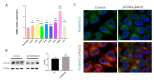
Rab21 is a GTPase protein that is functional in intracellular trafficking and involved in the pathologies of many diseases, such as Alzheimer's disease (AD), glioma, cancer, etc. Our previous work has reported its interaction with the catalytic subunit of gamma-secretase, PS1, and it regulates the activity of PS1 via transferring it from the early endosome to the late endosome/lysosome. However, it is still unknown how Rab21 protein itself is regulated. This work revealed that Rab21 protein, either endogenously or exogenously, can be degraded by the ubiquitin-proteasome pathway and the autophagy-lysosome pathway. It is further observed that the ubiquitinated Rab21 is increased, but the total protein is unchanged in AD model mice. We further observed that overexpression of Rab21 leads to increased expression of a series of genes involved in the autophagy-lysosome pathway. We speculated that even though the ubiquitinated Rab21 is increased due to the impaired proteasome function in the AD model, the autophagy-lysosome pathway functions in parallel to degrade Rab21 to keep its protein level in homeostasis. In conclusion, understanding the characters of Rab21 protein itself help explore its potential as a target for therapeutic strategy in diseases.
Keywords: Rab21 protein; autophagy-lysosome pathway; ubiquitin-proteasome pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Rab21, a Novel PS1 Interactor, Regulates γ-Secretase Activity via PS1 Subcellular Distribution.Mol Neurobiol. 2018 May;55(5):3841-3855. doi: 10.1007/s12035-017-0606-3. Epub 2017 May 25. Mol Neurobiol. 2018. PMID: 28547526
-
Starvation-induced MTMR13 and RAB21 activity regulates VAMP8 to promote autophagosome-lysosome fusion.EMBO Rep. 2015 Mar;16(3):297-311. doi: 10.15252/embr.201439464. Epub 2015 Feb 3. EMBO Rep. 2015. PMID: 25648148 Free PMC article.
-
Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system.Prog Neurobiol. 2013 Jun;105:49-59. doi: 10.1016/j.pneurobio.2013.03.001. Epub 2013 Mar 23. Prog Neurobiol. 2013. PMID: 23528736 Review.
-
Ubiquitin, Autophagy and Neurodegenerative Diseases.Cells. 2020 Sep 2;9(9):2022. doi: 10.3390/cells9092022. Cells. 2020. PMID: 32887381 Free PMC article. Review.
-
Degradation of regulator of calcineurin 1 (RCAN1) is mediated by both chaperone-mediated autophagy and ubiquitin proteasome pathways.FASEB J. 2009 Oct;23(10):3383-92. doi: 10.1096/fj.09-134296. Epub 2009 Jun 9. FASEB J. 2009. PMID: 19509306
Cited by
-
Upregulation of UBR1 m6A Methylation by METTL14 Inhibits Autophagy in Spinal Cord Injury.eNeuro. 2023 Jun 2;10(6):ENEURO.0338-22.2023. doi: 10.1523/ENEURO.0338-22.2023. Print 2023 Jun. eNeuro. 2023. PMID: 37094938 Free PMC article.
-
Cullin-3 regulates the renal baroreceptor machinery that controls renin gene expression.JCI Insight. 2025 Jul 8;10(15):e194075. doi: 10.1172/jci.insight.194075. eCollection 2025 Aug 8. JCI Insight. 2025. PMID: 40627460 Free PMC article.
-
Molecular mechanisms of mitochondrial homeostasis regulation in neurons and possible therapeutic approaches for Alzheimer's disease.Heliyon. 2024 Aug 17;10(17):e36470. doi: 10.1016/j.heliyon.2024.e36470. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281517 Free PMC article. Review.
-
RNF167 mediates atypical ubiquitylation and degradation of RLRs via two distinct proteolytic pathways.Nat Commun. 2025 Feb 24;16(1):1920. doi: 10.1038/s41467-025-57245-3. Nat Commun. 2025. PMID: 39994288 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

