Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans
- PMID: 35163054
- PMCID: PMC8835009
- DOI: 10.3390/ijms23031135
Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans
Abstract
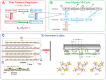
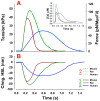
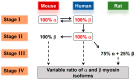
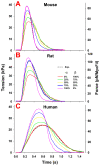
To understand how pathology-induced changes in contractile protein isoforms modulate cardiac muscle function, it is necessary to quantify the temporal-mechanical properties of contractions that occur under various conditions. Pathological responses are much easier to study in animal model systems than in humans, but extrapolation between species presents numerous challenges. Employing computational approaches can help elucidate relationships that are difficult to test experimentally by translating the observations from rats and mice, as model organisms, to the human heart. Here, we use the spatially explicit MUSICO platform to model twitch contractions from rodent and human trabeculae collected in a single laboratory. This approach allowed us to identify the variations in kinetic characteristics of α- and β-myosin isoforms across species and to quantify their effect on cardiac muscle contractile responses. The simulations showed how the twitch transient varied with the ratio of the two myosin isoforms. Particularly, the rate of tension rise was proportional to the fraction of α-myosin present, while the β-isoform dominated the rate of relaxation unless α-myosin was >50%. Moreover, both the myosin isoform and the Ca2+ transient contributed to the twitch tension transient, allowing two levels of regulation of twitch contraction.
Keywords: MUSICO platform; calcium sensitivity; cross-species simulation; level of incorporation; tension relaxation; trabeculae.
Conflict of interest statement
The authors declare no conflict of interest and this article reflects only the authors’ views. The European Commission is not responsible for any use that may be made of the information it contains.
Figures

Similar articles
-
Multiscale modeling of twitch contractions in cardiac trabeculae.J Gen Physiol. 2021 Mar 1;153(3):e202012604. doi: 10.1085/jgp.202012604. J Gen Physiol. 2021. PMID: 33512405 Free PMC article.
-
The force and stiffness of myosin motors in the isometric twitch of a cardiac trabecula and the effect of the extracellular calcium concentration.J Physiol. 2018 Jul;596(13):2581-2596. doi: 10.1113/JP275579. Epub 2018 May 27. J Physiol. 2018. PMID: 29714038 Free PMC article.
-
The effect of variable troponin C mutation thin filament incorporation on cardiac muscle twitch contractions.J Mol Cell Cardiol. 2021 Jun;155:112-124. doi: 10.1016/j.yjmcc.2021.02.009. Epub 2021 Feb 24. J Mol Cell Cardiol. 2021. PMID: 33636222 Free PMC article.
-
Investigations of Molecular Mechanisms of Actin-Myosin Interactions in Cardiac Muscle.Biochemistry (Mosc). 2015 Dec;80(13):1748-63. doi: 10.1134/S0006297915130106. Biochemistry (Mosc). 2015. PMID: 26878579 Review.
-
Alpha and beta myosin isoforms and human atrial and ventricular contraction.Cell Mol Life Sci. 2021 Dec;78(23):7309-7337. doi: 10.1007/s00018-021-03971-y. Epub 2021 Oct 26. Cell Mol Life Sci. 2021. PMID: 34704115 Free PMC article. Review.
Cited by
-
Computational Modeling on Drugs Effects for Left Ventricle in Cardiomyopathy Disease.Pharmaceutics. 2023 Feb 28;15(3):793. doi: 10.3390/pharmaceutics15030793. Pharmaceutics. 2023. PMID: 36986654 Free PMC article.
-
Myosin in autoinhibited off state(s), stabilized by mavacamten, can be recruited in response to inotropic interventions.Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2314914121. doi: 10.1073/pnas.2314914121. Epub 2024 Feb 12. Proc Natl Acad Sci U S A. 2024. PMID: 38346202 Free PMC article.
-
Proteomic profiling of sudden cardiac death with acquired cardiac hypertrophy.Int J Legal Med. 2023 Sep;137(5):1453-1461. doi: 10.1007/s00414-023-03038-6. Epub 2023 Jun 7. Int J Legal Med. 2023. PMID: 37284852 Free PMC article.
-
Machine learning meets Monte Carlo methods for models of muscle's molecular machinery to classify mutations.J Gen Physiol. 2023 May 1;155(5):e202213291. doi: 10.1085/jgp.202213291. Epub 2023 Mar 31. J Gen Physiol. 2023. PMID: 37000171 Free PMC article.
-
Myosins and MyomiR Network in Patients with Obstructive Hypertrophic Cardiomyopathy.Biomedicines. 2022 Sep 3;10(9):2180. doi: 10.3390/biomedicines10092180. Biomedicines. 2022. PMID: 36140281 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

