Retinal Plasticity
- PMID: 35163059
- PMCID: PMC8835074
- DOI: 10.3390/ijms23031138
Retinal Plasticity
Abstract
Brain plasticity is a well-established concept designating the ability of central nervous system (CNS) neurons to rearrange as a result of learning, when adapting to changeable environmental conditions or else while reacting to injurious factors. As a part of the CNS, the retina has been repeatedly probed for its possible ability to respond plastically to a variably altered environment or to pathological insults. However, numerous studies support the conclusion that the retina, outside the developmental stage, is endowed with only limited plasticity, exhibiting, instead, a remarkable ability to maintain a stable architectural and functional organization. Reviewed here are representative examples of hippocampal and cortical paradigms of plasticity and of retinal structural rearrangements found in organization and circuitry following altered developmental conditions or occurrence of genetic diseases leading to neuronal degeneration. The variable rate of plastic changes found in mammalian retinal neurons in different circumstances is discussed, focusing on structural plasticity. The likely adaptive value of maintaining a low level of plasticity in an organ subserving a sensory modality that is dominant for the human species and that requires elevated fidelity is discussed.
Keywords: deafferentation; remodeling; retinitis pigmentosa; structural plasticity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; the collection, analyses or interpretation of data; the writing of the manuscript; or in the decision to publish the results.
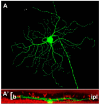
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

