Do We Have Viable Protective Strategies against Anesthesia-Induced Developmental Neurotoxicity?
- PMID: 35163060
- PMCID: PMC8834847
- DOI: 10.3390/ijms23031128
Do We Have Viable Protective Strategies against Anesthesia-Induced Developmental Neurotoxicity?
Abstract
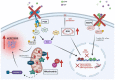
Since its invention, general anesthesia has been an indispensable component of modern surgery. While traditionally considered safe and beneficial in many pathological settings, hundreds of preclinical studies in various animal species have raised concerns about the detrimental and long-lasting consequences that general anesthetics may cause to the developing brain. Clinical evidence of anesthetic neurotoxicity in humans continues to mount as we continue to contemplate how to move forward. Notwithstanding the alarming evidence, millions of children are being anesthetized each year, setting the stage for substantial healthcare burdens in the future. Hence, furthering our knowledge of the molecular underpinnings of anesthesia-induced developmental neurotoxicity is crucially important and should enable us to develop protective strategies so that currently available general anesthetics could be safely used during critical stages of brain development. In this mini-review, we provide a summary of select strategies with primary focus on the mechanisms of neuroprotection and potential for clinical applicability. First, we summarize a diverse group of chemicals with the emphasis on intracellular targets and signal-transduction pathways. We then discuss epigenetic and transgenerational effects of general anesthetics and potential remedies, and also anesthesia-sparing or anesthesia-delaying approaches. Finally, we present evidence of a novel class of anesthetics with a distinct mechanism of action and a promising safety profile.
Keywords: ROS; mitochondria; neonatal anesthesia; neuroactive steroids; neuroprotection; neurotoxicity; signaling pathways.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Molecular pathways of mitochondrial dysfunctions: possible cause of cell death in anesthesia-induced developmental neurotoxicity.Brain Res Bull. 2015 Jan;110:14-9. doi: 10.1016/j.brainresbull.2014.10.011. Epub 2014 Oct 28. Brain Res Bull. 2015. PMID: 25451455 Review.
-
Neurotoxicity of anesthetics: Mechanisms and meaning from mouse intervention studies.Neurotoxicol Teratol. 2019 Jan-Feb;71:22-31. doi: 10.1016/j.ntt.2018.11.004. Epub 2018 Nov 22. Neurotoxicol Teratol. 2019. PMID: 30472095 Free PMC article. Review.
-
Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity.Neurotoxicol Teratol. 2017 Mar-Apr;60:2-23. doi: 10.1016/j.ntt.2016.11.005. Epub 2016 Nov 18. Neurotoxicol Teratol. 2017. PMID: 27871903 Review.
-
Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system.Anesth Analg. 2008 Jun;106(6):1643-58. doi: 10.1213/ane.ob013e3181732c01. Anesth Analg. 2008. PMID: 18499593 Review.
-
Anesthesia and the developing brain: A way forward for laboratory and clinical research.Paediatr Anaesth. 2018 Sep;28(9):758-763. doi: 10.1111/pan.13455. Epub 2018 Aug 16. Paediatr Anaesth. 2018. PMID: 30117228 Review.
Cited by
-
Integrated Excitatory/Inhibitory Imbalance and Transcriptomic Analysis Reveals the Association between Dysregulated Synaptic Genes and Anesthetic-Induced Cognitive Dysfunction.Cells. 2022 Aug 11;11(16):2497. doi: 10.3390/cells11162497. Cells. 2022. PMID: 36010580 Free PMC article.
-
Brain injury and long-term outcome after neonatal surgery for non-cardiac congenital anomalies.Pediatr Res. 2023 Oct;94(4):1265-1272. doi: 10.1038/s41390-023-02629-8. Epub 2023 May 22. Pediatr Res. 2023. PMID: 37217607
-
Nonapoptotic caspases in neural development and in anesthesia-induced neurotoxicity.Trends Neurosci. 2022 Jun;45(6):446-458. doi: 10.1016/j.tins.2022.03.007. Epub 2022 Apr 28. Trends Neurosci. 2022. PMID: 35491256 Free PMC article. Review.
-
The persistent effects of anaesthesia on the brain.BJA Educ. 2023 Aug;23(8):304-311. doi: 10.1016/j.bjae.2023.04.001. Epub 2023 Jun 7. BJA Educ. 2023. PMID: 37465234 Free PMC article. Review. No abstract available.
-
Triggering Receptor Expressed on Myeloid Cells 2 Alleviated Sevoflurane-Induced Developmental Neurotoxicity via Microglial Pruning of Dendritic Spines in the CA1 Region of the Hippocampus.Neurosci Bull. 2024 Sep;40(9):1215-1229. doi: 10.1007/s12264-024-01260-9. Epub 2024 Jul 29. Neurosci Bull. 2024. PMID: 39078595
References
-
- Jevtovic-Todorovic V., Hartman R.E., Izumi Y., Benshoff N.D., Dikranian K., Zorumski C.F., Olney J.W., Wozniak D.F. Early Exposure to Common Anesthetic Agents Causes Widespread Neurodegeneration in the Developing Rat Brain and Persistent Learning Deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. - DOI - PMC - PubMed
-
- Paule M., Li M., Allen R., Liu F., Zou X., Hotchkiss C., Hanig J., Patterson T., Slikker W., Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol. Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. - DOI - PMC - PubMed
-
- Schenning K.J., Noguchi K.K., Martin L.D., Manzella F.M., Cabrera O.H., Dissen G.A., Brambrink A.M. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol. Teratol. 2017;60:63–68. doi: 10.1016/j.ntt.2016.11.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

