Paclitaxel-Induced Epidermal Alterations: An In Vitro Preclinical Assessment in Primary Keratinocytes and in a 3D Epidermis Model
- PMID: 35163066
- PMCID: PMC8834980
- DOI: 10.3390/ijms23031142
Paclitaxel-Induced Epidermal Alterations: An In Vitro Preclinical Assessment in Primary Keratinocytes and in a 3D Epidermis Model
Abstract
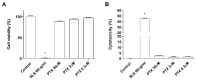
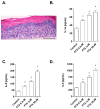
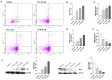
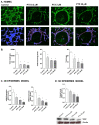
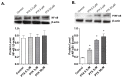
Paclitaxel is a microtubule-stabilizing chemotherapeutic agent approved for the treatment of ovarian, non-small cell lung, head, neck, and breast cancers. Despite its beneficial effects on cancer and widespread use, paclitaxel also damages healthy tissues, including the skin. However, the mechanisms that drive these skin adverse events are not clearly understood. In the present study, we demonstrated, by using both primary epidermal keratinocytes (NHEK) and a 3D epidermis model, that paclitaxel impairs different cellular processes: paclitaxel increased the release of IL-1α, IL-6, and IL-8 inflammatory cytokines, produced reactive oxygen species (ROS) release and apoptosis, and reduced the endothelial tube formation in the dermal microvascular endothelial cells (HDMEC). Some of the mechanisms driving these adverse skin events in vitro are mediated by the activation of toll-like receptor 4 (TLR-4), which phosphorylate transcription of nuclear factor kappa B (NF-κb). This is the first study analyzing paclitaxel effects on healthy human epidermal cells with an epidermis 3D model, and will help in understanding paclitaxel's effects on the skin.
Keywords: 3D epidermis model; NHEK; epidermis; paclitaxel.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Cell type-specific regulatory effects of glucocorticoids on cutaneous TLR2 expression and signalling.J Steroid Biochem Mol Biol. 2017 Jul;171:201-208. doi: 10.1016/j.jsbmb.2017.03.023. Epub 2017 Apr 2. J Steroid Biochem Mol Biol. 2017. PMID: 28377308
-
Pomegranate fruit extract modulates UV-B-mediated phosphorylation of mitogen-activated protein kinases and activation of nuclear factor kappa B in normal human epidermal keratinocytes paragraph sign.Photochem Photobiol. 2005 Jan-Feb;81(1):38-45. doi: 10.1562/2004-08-06-RA-264. Photochem Photobiol. 2005. PMID: 15493960
-
Apigenin reduces the Toll-like receptor-4-dependent activation of NF-κB by suppressing the Akt, mTOR, JNK, and p38-MAPK.Naunyn Schmiedebergs Arch Pharmacol. 2018 Mar;391(3):271-283. doi: 10.1007/s00210-017-1454-4. Epub 2017 Dec 20. Naunyn Schmiedebergs Arch Pharmacol. 2018. PMID: 29264665
-
Candida albicans phospholipomannan triggers inflammatory responses of human keratinocytes through Toll-like receptor 2.Exp Dermatol. 2009 Jul;18(7):603-10. doi: 10.1111/j.1600-0625.2008.00832.x. Epub 2008 Dec 19. Exp Dermatol. 2009. PMID: 19196344
-
Reduced Nrf2 activation in PI3K phosphorylation-impaired vitiliginous keratinocytes increases susceptibility to ROS-generating chemical-induced apoptosis.Environ Toxicol. 2017 Dec;32(12):2481-2491. doi: 10.1002/tox.22461. Epub 2017 Aug 24. Environ Toxicol. 2017. PMID: 28836394
Cited by
-
Transgenic zebrafish embryos to evaluate the in vivo effects of different liposome-paclitaxel nanocarrier system.Sci Rep. 2025 May 26;15(1):18358. doi: 10.1038/s41598-025-00258-1. Sci Rep. 2025. PMID: 40419515 Free PMC article.
-
Phosphodiesterase 4 is overexpressed in keloid epidermal scars and its inhibition reduces keratinocyte fibrotic alterations.Mol Med. 2024 Sep 2;30(1):134. doi: 10.1186/s10020-024-00906-8. Mol Med. 2024. PMID: 39223490 Free PMC article.
-
Molecular Research and Treatment of Skin Diseases.Int J Mol Sci. 2022 May 13;23(10):5435. doi: 10.3390/ijms23105435. Int J Mol Sci. 2022. PMID: 35628246 Free PMC article.
-
The Protective Anticancer Effect of Natural Lycopene Supercritical CO2 Watermelon Extracts in Adenocarcinoma Lung Cancer Cells.Antioxidants (Basel). 2022 Jun 11;11(6):1150. doi: 10.3390/antiox11061150. Antioxidants (Basel). 2022. PMID: 35740047 Free PMC article.
-
Synergistic celecoxib and dimethyl-celecoxib combinations block cervix cancer growth through multiple mechanisms.PLoS One. 2024 Sep 26;19(9):e0308233. doi: 10.1371/journal.pone.0308233. eCollection 2024. PLoS One. 2024. PMID: 39325741 Free PMC article.
References
-
- Alqahtani F.Y., Aleanizy F.S., El Tahir E., Alkahtani H.M., AlQuadeib B.T. Chapter three—Paclitaxel. In: Brittain H.G., editor. Profiles of Drug Substances, Excipients and Related Methodology. Academic Press; London, UK: 2019. pp. 205–238. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

