Gal3 Plays a Deleterious Role in a Mouse Model of Endotoxemia
- PMID: 35163089
- PMCID: PMC8835800
- DOI: 10.3390/ijms23031170
Gal3 Plays a Deleterious Role in a Mouse Model of Endotoxemia
Abstract
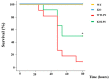
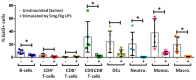
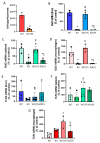
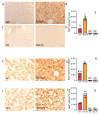
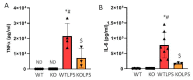
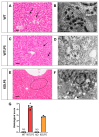
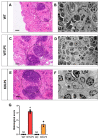
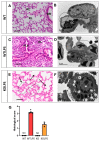
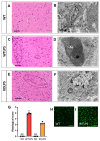
Lipopolysaccharide (LPS)-induced endotoxemia induces an acute systemic inflammatory response that mimics some important features of sepsis, the disease with the highest mortality rate worldwide. In this work, we have analyzed a murine model of endotoxemia based on a single intraperitoneal injection of 5 mg/kg of LPS. We took advantage of galectin-3 (Gal3) knockout mice and found that the absence of Gal3 decreased the mortality rate oflethal endotoxemia in the first 80 h after the administration of LPS, along with a reduction in the tissular damage in several organs measured by electron microscopy. Using flow cytometry, we demonstrated that, in control conditions, peripheral immune cells, especially monocytes, exhibited high levels of Gal3, which were early depleted in response to LPS injection, thus suggesting Gal3 release under endotoxemia conditions. However, serum levels of Gal3 early decreased in response to LPS challenge (1 h), an indication that Gal3 may be extravasated to peripheral organs. Indeed, analysis of Gal3 in peripheral organs revealed a robust up-regulation of Gal3 36 h after LPS injection. Taken together, these results demonstrate the important role that Gal3 could play in the development of systemic inflammation, a well-established feature of sepsis, thus opening new and promising therapeutic options for these harmful conditions.
Keywords: electron microscopy; endotoxemia; galectin-3; lipopolysaccharide; sepsis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
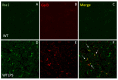
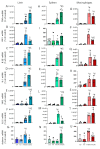
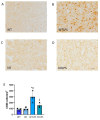
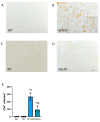
Similar articles
-
Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses.Am J Pathol. 2000 Mar;156(3):1073-83. doi: 10.1016/S0002-9440(10)64975-9. Am J Pathol. 2000. PMID: 10702423 Free PMC article.
-
Galectin-3 is critical for the development of the allergic inflammatory response in a mouse model of atopic dermatitis.Am J Pathol. 2009 Mar;174(3):922-31. doi: 10.2353/ajpath.2009.080500. Epub 2009 Jan 29. Am J Pathol. 2009. PMID: 19179612 Free PMC article.
-
Galectin-3 mediates survival and apoptosis pathways during Trypanosoma cruzi-host cell interplay.Exp Parasitol. 2020 Sep;216:107932. doi: 10.1016/j.exppara.2020.107932. Epub 2020 Jun 12. Exp Parasitol. 2020. PMID: 32535113
-
Multiorgan Development of Oxidative and Nitrosative Stress in LPS-Induced Endotoxemia in C57Bl/6 Mice: DHE-Based In Vivo Approach.Oxid Med Cell Longev. 2019 May 22;2019:7838406. doi: 10.1155/2019/7838406. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31249650 Free PMC article.
-
Participation of Monocyte Subpopulations in Progression of Experimental Endotoxemia (EE) and Systemic Inflammation.J Immunol Res. 2021 Feb 12;2021:1762584. doi: 10.1155/2021/1762584. eCollection 2021. J Immunol Res. 2021. PMID: 33628841 Free PMC article. Review.
Cited by
-
Inflammasome and pyroptosis in autoimmune liver diseases.Front Immunol. 2023 Mar 8;14:1150879. doi: 10.3389/fimmu.2023.1150879. eCollection 2023. Front Immunol. 2023. PMID: 36969233 Free PMC article. Review.
-
Processed Dietary Fiber Partially Hydrolyzed Guar Gum Increases Susceptibility to Colitis and Colon Tumorigenesis in Mice.Res Sq [Preprint]. 2024 Dec 9:rs.3.rs-5522559. doi: 10.21203/rs.3.rs-5522559/v1. Res Sq. 2024. Update in: NPJ Sci Food. 2025 Jul 1;9(1):114. doi: 10.1038/s41538-025-00472-1. PMID: 39711544 Free PMC article. Updated. Preprint.
-
Serum galectin-3 levels predict poor prognosis in sepsis and septic shock patients.Rev Assoc Med Bras (1992). 2023 Aug 21;69(8):e20220940. doi: 10.1590/1806-9282.20220940. eCollection 2023. Rev Assoc Med Bras (1992). 2023. PMID: 37610926 Free PMC article.
-
Dietary fiber guar gum-induced shift in gut microbiota metabolism and intestinal immune activity enhances susceptibility to colonic inflammation.Gut Microbes. 2024 Jan-Dec;16(1):2341457. doi: 10.1080/19490976.2024.2341457. Epub 2024 Apr 17. Gut Microbes. 2024. PMID: 38630030 Free PMC article.
-
Processed dietary fiber partially hydrolyzed guar gum increases susceptibility to colitis and colon tumorigenesis in mice.NPJ Sci Food. 2025 Jul 1;9(1):114. doi: 10.1038/s41538-025-00472-1. NPJ Sci Food. 2025. PMID: 40595638 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

