Evidence for Inhibitory Perturbations on the Amplitude, Gating, and Hysteresis of A-Type Potassium Current, Produced by Lacosamide, a Functionalized Amino Acid with Anticonvulsant Properties
- PMID: 35163091
- PMCID: PMC8835568
- DOI: 10.3390/ijms23031171
Evidence for Inhibitory Perturbations on the Amplitude, Gating, and Hysteresis of A-Type Potassium Current, Produced by Lacosamide, a Functionalized Amino Acid with Anticonvulsant Properties
Abstract
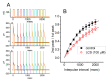
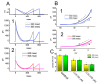
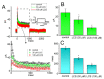
Lacosamide (Vimpat®, LCS) is widely known as a functionalized amino acid with promising anti-convulsant properties; however, adverse events during its use have gradually appeared. Despite its inhibitory effect on voltage-gated Na+ current (INa), the modifications on varying types of ionic currents caused by this drug remain largely unexplored. In pituitary tumor (GH3) cells, we found that the presence of LCS concentration-dependently decreased the amplitude of A-type K+ current (IK(A)) elicited in response to membrane depolarization. The IK(A) amplitude in these cells was sensitive to attenuation by the application of 4-aminopyridine, 4-aminopyridine-3-methanol, or capsaicin but not by that of tetraethylammonium chloride. The effective IC50 value required for its reduction in peak or sustained IK(A) was calculated to be 102 or 42 µM, respectively, while the value of the dissociation constant (KD) estimated from the slow component in IK(A) inactivation at varying LCS concentrations was 52 µM. By use of two-step voltage protocol, the presence of this drug resulted in a rightward shift in the steady-state inactivation curve of IK(A) as well as in a slowing in the recovery time course of the current block; however, no change in the gating charge of the inactivation curve was detected in its presence. Moreover, the LCS addition led to an attenuation in the degree of voltage-dependent hysteresis for IK(A) elicitation by long-duration triangular ramp voltage commands. Likewise, the IK(A) identified in mouse mHippoE-14 neurons was also sensitive to block by LCS, coincident with an elevation in the current inactivation rate. Collectively, apart from its canonical action on INa inhibition, LCS was effective at altering the amplitude, gating, and hysteresis of IK(A) in excitable cells. The modulatory actions on IK(A), caused by LCS, could interfere with the functional activities of electrically excitable cells (e.g., pituitary tumor cells or hippocampal neurons).
Keywords: A-type K+ current; current kinetics; hippocampal neuron; lacosamide (Vimpat®); pituitary cell; voltage hysteresis.
Conflict of interest statement
The authors declare no conflict of interest that is directly relevant to this study.
Figures
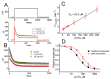
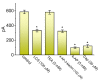
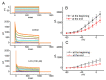
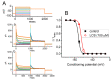
References
-
- Ortiz de la Rosa J.S., Ladino L.D., Rodríguez P.J., Rueda M.C., Polanía J.P., Castañeda A.C. Efficacy of lacosamide in children and adolescents with drug-resistant epilepsy and refractory status epilepticus: A systematic review. Seizure. 2018;56:34–40. doi: 10.1016/j.seizure.2018.01.014. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

