Protein Dimerization via Tyr Residues: Highlight of a Slow Process with Co-Existence of Numerous Intermediates and Final Products
- PMID: 35163094
- PMCID: PMC8835203
- DOI: 10.3390/ijms23031174
Protein Dimerization via Tyr Residues: Highlight of a Slow Process with Co-Existence of Numerous Intermediates and Final Products
Abstract
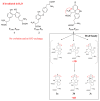
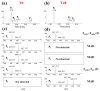
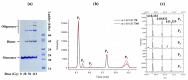
Protein dimerization via tyrosine residues is a crucial process in response to an oxidative attack, which has been identified in many ageing-related pathologies. Recently, it has been found that for isolated tyrosine amino acid, dimerization occurs through three types of tyrosine-tyrosine crosslinks and leads to at least four final products. Herein, considering two protected tyrosine residues, tyrosine-containing peptides and finally proteins, we investigate the dimerization behavior of tyrosine when embedded in a peptidic sequence. After azide radical oxidation and by combining UPLC-MS and H/D exchange analyzes, we were able to evidence: (i) the slow kinetics of Michael Addition Dimers (MAD) formation, i.e., more than 48 h; (ii) the co-existence of intermediates and final cyclized dimer products; and (iii) the probable involvement of amide functions to achieve Michael additions even in proteins. This raises the question of the possible in vivo existence of both intermediates and final entities as well as their toxicity and the potential consequences on protein structure and/or function.
Keywords: oxidative stress; proteins; radicals; tyrosine dimers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
How protein structure affects redox reactivity: example of Human centrin 2.Phys Chem Chem Phys. 2014 Nov 28;16(44):24493-8. doi: 10.1039/c4cp03536d. Phys Chem Chem Phys. 2014. PMID: 25308418
-
Aggregation of α- and β- caseins induced by peroxyl radicals involves secondary reactions of carbonyl compounds as well as di-tyrosine and di-tryptophan formation.Free Radic Biol Med. 2018 Aug 20;124:176-188. doi: 10.1016/j.freeradbiomed.2018.06.005. Epub 2018 Jun 7. Free Radic Biol Med. 2018. PMID: 29885785
-
Oxidative stress induces mainly human centrin 2 polymerisation.Int J Radiat Biol. 2010 Aug;86(8):657-68. doi: 10.3109/09553001003734584. Int J Radiat Biol. 2010. PMID: 20586543
-
3-Nitrotyrosine and related derivatives in proteins: precursors, radical intermediates and impact in function.Essays Biochem. 2020 Feb 17;64(1):111-133. doi: 10.1042/EBC20190052. Essays Biochem. 2020. PMID: 32016371 Review.
-
Protein tyrosine nitration in hydrophilic and hydrophobic environments.Amino Acids. 2007;32(4):501-15. doi: 10.1007/s00726-006-0425-8. Epub 2006 Nov 2. Amino Acids. 2007. PMID: 17077966 Review.
Cited by
-
From data extraction to analysis: a comparative study of ELISE capabilities in scientific literature.Front Artif Intell. 2025 May 12;8:1587244. doi: 10.3389/frai.2025.1587244. eCollection 2025. Front Artif Intell. 2025. PMID: 40420940 Free PMC article.
-
Insights into heme degradation and hydrogen peroxide-induced dimerization of human neuroglobin.Biosci Rep. 2025 Jan 30;45(1):1-13. doi: 10.1042/BSR20241265. Biosci Rep. 2025. PMID: 39631057 Free PMC article.
-
Photoradical-Mediated Catalyst-Independent Protein Cross-Link with Unusual Fluorescence Properties.Chembiochem. 2023 Sep 1;24(17):e202300380. doi: 10.1002/cbic.202300380. Epub 2023 Aug 1. Chembiochem. 2023. PMID: 37232210 Free PMC article.
-
Light from Afield: Fast, High-Resolution, and Layer-Free Deep Vat 3D Printing.Chem Rev. 2024 Jul 24;124(14):8787-8822. doi: 10.1021/acs.chemrev.4c00134. Epub 2024 Jul 5. Chem Rev. 2024. PMID: 38967405 Free PMC article. Review.
-
Spontaneous and Ionizing Radiation-Induced Aggregation of Human Serum Albumin: Dityrosine as a Fluorescent Probe.Int J Mol Sci. 2022 Jul 22;23(15):8090. doi: 10.3390/ijms23158090. Int J Mol Sci. 2022. PMID: 35897662 Free PMC article.
References
-
- Chatterjee S. Oxidative Stress and Biomaterials. Elsevier; Amsterdam, The Netherlands: 2016. Oxidative Stress, Inflammation, and Disease; pp. 35–58.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

