Induced Neurodifferentiation of hBM-MSCs through Activation of the ERK/CREB Pathway via Pulsed Electromagnetic Fields and Physical Stimulation Promotes Neurogenesis in Cerebral Ischemic Models
- PMID: 35163096
- PMCID: PMC8835447
- DOI: 10.3390/ijms23031177
Induced Neurodifferentiation of hBM-MSCs through Activation of the ERK/CREB Pathway via Pulsed Electromagnetic Fields and Physical Stimulation Promotes Neurogenesis in Cerebral Ischemic Models
Abstract
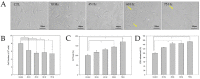
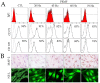
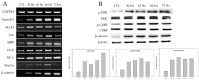
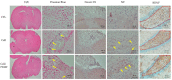
Stroke is among the leading causes of death worldwide, and stroke patients are more likely to live with permanent disabilities even after treatment. Several treatments are being developed to improve the quality of life of patients; however, these treatments still have important limitations. Our study thus sought to evaluate the neural differentiation of human bone marrow mesenchymal stem cells (hBM-MSCs) at various pulsed electromagnetic field (PEMF) frequencies. Furthermore, the effects of selected frequencies in vivo were also evaluated using a mouse ischemia stroke model. Cell proliferation decreased by 20% in the PEMF group, as demonstrated by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay, and lactate dehydrogenase (LDH) secretion increased by approximately 10% in an LDH release assay. Fluorescence-activated cell sorting (FACS) analysis demonstrated that CD73 and CD105 were downregulated in the PEMF group at 60 Hz. Moreover, microtubule-associated protein 2 (MAP-2) and neurofilament light chain (NF-L) were upregulated in cell cultures at 60 and 75 Hz. To assess the effects of PEMF in vivo, cerebral ischemia mice were exposed to a PEMF at 60 Hz. Neural-related proteins were significantly upregulated in the PEMF groups compared with the control and cell group. Upon conducting rotarod tests, the cell/PEMF group exhibited significant differences in motor coordination at 13 days post-treatment when compared with the control and stem-cell-treated group. Furthermore, the cell and cell/PEMF group exhibited a significant reduction in the expression of matrix metalloproteinase-9 (MMP-9), tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) in the induced ischemic area compared with the control. Collectively, our findings demonstrated that PEMFs at 60 and 75 Hz could stimulate hBM-MSCs neural differentiation in vitro, in addition to promoting neurogenesis to enhance the functional recovery process by reducing the post-stroke inflammatory reaction.
Keywords: cerebral ischemia stroke; inflammatory cytokines; neural differentiation; pulse electromagnetic fields.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Avan A., Digaleh H., Di Napoli M., Stranges S., Behrouz R., Shojaeianbabaei G., Amiri A., Tabrizi R., Mokhber N., Spence J.D., et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17:191. doi: 10.1186/s12916-019-1397-3. - DOI - PMC - PubMed
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

