The Apoptosis Paradox in Cancer
- PMID: 35163253
- PMCID: PMC8836235
- DOI: 10.3390/ijms23031328
The Apoptosis Paradox in Cancer
Abstract
Cancer growth represents a dysregulated imbalance between cell gain and cell loss, where the rate of proliferating mutant tumour cells exceeds the rate of those that die. Apoptosis, the most renowned form of programmed cell death, operates as a key physiological mechanism that limits cell population expansion, either to maintain tissue homeostasis or to remove potentially harmful cells, such as those that have sustained DNA damage. Paradoxically, high-grade cancers are generally associated with high constitutive levels of apoptosis. In cancer, cell-autonomous apoptosis constitutes a common tumour suppressor mechanism, a property which is exploited in cancer therapy. By contrast, limited apoptosis in the tumour-cell population also has the potential to promote cell survival and resistance to therapy by conditioning the tumour microenvironment (TME)-including phagocytes and viable tumour cells-and engendering pro-oncogenic effects. Notably, the constitutive apoptosis-mediated activation of cells of the innate immune system can help orchestrate a pro-oncogenic TME and may also effect evasion of cancer treatment. Here, we present an overview of the implications of cell death programmes in tumour biology, with particular focus on apoptosis as a process with "double-edged" consequences: on the one hand, being tumour suppressive through deletion of malignant or pre-malignant cells, while, on the other, being tumour progressive through stimulation of reparatory and regenerative responses in the TME.
Keywords: apoptosis; cancer; cancer therapy; cell death; extracellular vesicle; immune system; macrophage; regeneration; repair; tumour.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
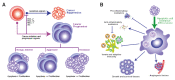
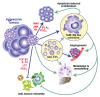
References
-
- Green D.R. Cell Death. Apoptosis and Other Means to an End. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2018.
-
- Jalalinadoushan M., Peivareh H., Azizzadeh Delshad A. Correlation between Apoptosis and Histological Grade of Transitional Cell Carcinoma of Urinary Bladder. Urol. J. 2004;1:177–179. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

