Curcumin Loaded Nanocarriers with Varying Charges Augmented with Electroporation Designed for Colon Cancer Therapy
- PMID: 35163301
- PMCID: PMC8836164
- DOI: 10.3390/ijms23031377
Curcumin Loaded Nanocarriers with Varying Charges Augmented with Electroporation Designed for Colon Cancer Therapy
Abstract
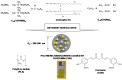
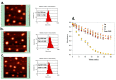
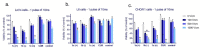
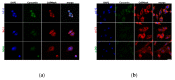
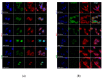
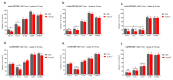
(1) Background: The size and surface charge are the most significant parameters of nanocarriers that determine their efficiency and potential application. The poor cell uptake of encapsulated drugs is the main limitation in anticancer treatment. The well-defined properties of nanocarriers will enable to target specific tissue and deliver an active cargo. (2) Methods: In the current study, poly(D,L -lactide) (PLA) nanocarriers loaded with curcumin (CUR) and differing surface charge were evaluated for transport efficacy in combination with electroporation (EP) in dependence on the type of cells. The obtained CUR-loaded nanoparticles with diameters ranging from 195 to 334 nm (derived from dynamic light scattering (DLS)) were characterized by atomic force microscopy (AFM) (morphology and shape) and Doppler electrophoresis (ζ-potential) as well as UV-vis spectroscopy (CUR encapsulation efficiency (about 90%) and photobleaching rate). The drug delivery properties of the obtained PLA nanocarriers enhanced by electroporation were assessed in human colon cancer cells (LoVo), excitable normal rat muscle cells (L6), and free of voltage-gated ion channels cells (CHO-K1). CLSM studies, viability, and ROS release were performed to determine the biological effects of nanocarriers. (3) Results: The highest photodynamic activity indicated anionic nanocarriers (1a) stabilized by C12(COONa)2 surfactant. Nanocarriers were cytotoxic for LoVo cells and less cytotoxic for normal cells. ROS release increased in cancer cells with the increasing electric field intensity, irradiation, and time after EP. Muscle L6 cells were less sensitive to electric pulses. (4) Conclusions: EP stimulation for CUR-PLA nanocarriers transport was considered to improve the regulated and more effective delivery of nanosystems differing in surface charge.
Keywords: PLA nanocarriers; colon cancer; curcumin; electroporation; surface charge.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ruiz De Porras V., Bystrup S., Martínez-Cardús A., Pluvinet R., Sumoy L., Howells L., James M.I., Iwuji C., Manzano J.L., Layos L., et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci. Rep. 2016;6:24675. doi: 10.1038/srep24675. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

