pH-Sensing G Protein-Coupled Receptor OGR1 (GPR68) Expression and Activation Increases in Intestinal Inflammation and Fibrosis
- PMID: 35163345
- PMCID: PMC8835966
- DOI: 10.3390/ijms23031419
pH-Sensing G Protein-Coupled Receptor OGR1 (GPR68) Expression and Activation Increases in Intestinal Inflammation and Fibrosis
Abstract
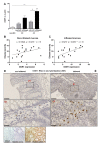
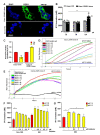
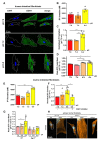
Local extracellular acidification occurs at sites of inflammation. Proton-sensing ovarian cancer G-protein-coupled receptor 1 (OGR1, also known as GPR68) responds to decreases in extracellular pH. Our previous studies show a role for OGR1 in the pathogenesis of mucosal inflammation, suggesting a link between tissue pH and immune responses. Additionally, pH-dependent signalling is associated with the progression of intestinal fibrosis. In this study, we aimed to investigate OGR1 expression and OGR1-mediated signalling in patients with inflammatory bowel disease (IBD). Our results show that OGR1 expression significantly increased in patients with IBD compared to non-IBD patients, as demonstrated by qPCR and immunohistochemistry (IHC). Paired samples from non-inflamed and inflamed intestinal areas of IBD patients showed stronger OGR1 IHC staining in inflamed mucosal segments compared to non-inflamed mucosa. IHC of human surgical samples revealed OGR1 expression in macrophages, granulocytes, endothelial cells, and fibroblasts. OGR1-dependent inositol phosphate (IP) production was significantly increased in CD14+ monocytes from IBD patients compared to healthy subjects. Primary human and murine fibroblasts exhibited OGR1-dependent IP formation, RhoA activation, F-actin, and stress fibre formation upon an acidic pH shift. OGR1 expression and signalling increases with IBD disease activity, suggesting an active role of OGR1 in the pathogenesis of IBD.
Keywords: OGR1 (GPR68) expression and function; fibroblasts; fibrosis; inflammatory bowel disease; pH-sensing GPCR.
Conflict of interest statement
G.R. discloses grant support from AbbVie, Ardeypharm, MSD, FALK, Flamentera, Novartis, Roche, Tillots, UCB, and Zeller. M.H. discloses grant support from AbbVie and Novartis. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Krawczyk C.M., Holowka T., Sun J., Blagih J., Amiel E., DeBerardinis R.J., Cross J.R., Jung E., Thompson C.B., Jones R.G., et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. - DOI - PMC - PubMed
-
- Lardner A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001;69:522–530. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

