A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation
- PMID: 35163348
- PMCID: PMC8835857
- DOI: 10.3390/ijms23031425
A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation
Abstract
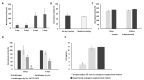
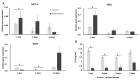
The clinical breakthrough of bone tissue engineering (BTE) depends on the ability to provide patients routinely with BTE products of consistent pharmacological quality. The bottleneck of this approach is the availability of stem cells. To avoid this, we suggest immobilization of random-donor-derived heterologous osteoinductive MSCs onto osteoconductive matrices. Such BTE products could then be frozen and, after thawing, could be released as ready-to-use products for permanent implantation during surgery. For this purpose, we developed a simple protocol for cryopreservation of BTE constructs and evaluated the effects of this procedure on human MSC (hMSCs) metabolic and osteogenic activity in vitro. Our findings show that hMSCs can be freeze-thawed on a β-TCP scaffold through a technically simple procedure. Treated cells sustained their metabolic activity and showed favorable osteogenic potential. Mechanistically, HIF1α and YBX1 genes were activated after freeze-thawing, and supposed to be linked to enhanced osteogenesis. However, the detailed mechanisms as to how the cryopreservation procedure beneficially affects the osteogenic potential of hMSCs remains to be evaluated. Additionally, we demonstrated that our BTE products could be stored for 3 days on dry ice; this could facilitate the supply chain management of cryopreserved BTE constructs from the site of manufacture to the operating room.
Keywords: 3D culture; cryopreservation; human MSCs; osteogenic differentiation; scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

