Ten Approaches That Improve Immunostaining: A Review of the Latest Advances for the Optimization of Immunofluorescence
- PMID: 35163349
- PMCID: PMC8836139
- DOI: 10.3390/ijms23031426
Ten Approaches That Improve Immunostaining: A Review of the Latest Advances for the Optimization of Immunofluorescence
Abstract
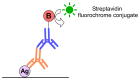
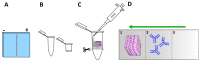
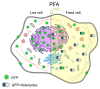
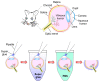
Immunostaining has emerged as one of the most common and valuable techniques that allow the localization of proteins at a quantitative level within cells and tissues using antibodies coupled to enzymes, fluorochromes, or colloidal nanogold particles. The application of fluorochromes during immunolabeling is referred to as immunofluorescence, a method coupled to widefield or confocal microscopy and extensively applied in basic research and clinical diagnosis. Notwithstanding, there are still disadvantages associated with the application of this technique due to technical challenges in the process, such as sample fixation, permeabilization, antibody incubation times, and fluid exchange, etc. These disadvantages call for continuous updates and improvements to the protocols extensively described in the literature. This review contributes to protocol optimization, outlining 10 current methods for improving sample processing in different stages of immunofluorescence, including a section with further recommendations. Additionally, we have extended our own antibody signal enhancer method, which was reported to significantly increase antibody signals and is useful for cervical cancer detection, to improve the signals of fluorochrome-conjugated staining reagents in fibrous tissues. In summary, this review is a valuable tool for experienced researchers and beginners when planning or troubleshooting the immunofluorescence assay.
Keywords: antibody; immunocytofluorescence; immunohistofluorescence; immunolabeling; immunolocalization; signal-to-noise ratio; unmask epitopes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Flores-Maldonado C., Albino-Sánchez M.E., Rodríguez-Callejas J.D., Estrada-Mondragon A., León-Galicia I., Maqueda-Alfaro R., Perez-Cruz C., Fuchs E., García-Carrancá A., Contreras R.G., et al. A Low Cost Antibody Signal Enhancer Improves Immunolabeling in Cell Culture, Primate Brain and Human Cancer Biopsy. Neuroscience. 2020;439:275–286. doi: 10.1016/j.neuroscience.2020.01.009. - DOI - PubMed
-
- Childs G.V. History of Immunohistochemistry. Pathobiol. Hum. Dis. A Dyn. Encycl. Dis. Mech. 2014:3775–3796. doi: 10.1016/B978-0-12-386456-7.07401-3. - DOI
-
- Sternberger L.A., Hardy P.H., Cuculis J.J., Meyer H.G. The unlabeled antibody enzyme method of immunohistochemistry: Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J. Histochem. Cytochem. 1970;18:315–333. doi: 10.1177/18.5.315. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

