Integrin Signaling in the Central Nervous System in Animals and Human Brain Diseases
- PMID: 35163359
- PMCID: PMC8836133
- DOI: 10.3390/ijms23031435
Integrin Signaling in the Central Nervous System in Animals and Human Brain Diseases
Abstract
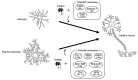
The integrin family is involved in various biological functions, including cell proliferation, differentiation and migration, and also in the pathogenesis of disease. Integrins are multifunctional receptors that exist as heterodimers composed of α and β subunits and bind to various ligands, including extracellular matrix (ECM) proteins; they are found in many animals, not only vertebrates (e.g., mouse, rat, and teleost fish), but also invertebrates (e.g., planarian flatworm, fruit fly, nematodes, and cephalopods), which are used for research on genetics and social behaviors or as models for human diseases. In the present paper, we describe the results of a phylogenetic tree analysis of the integrin family among these species. We summarize integrin signaling in teleost fish, which serves as an excellent model for the study of regenerative systems and possesses the ability for replacing missing tissues, especially in the central nervous system, which has not been demonstrated in mammals. In addition, functions of astrocytes and reactive astrocytes, which contain neuroprotective subpopulations that act in concert with the ECM proteins tenascin C and osteopontin via integrin are also reviewed. Drug development research using integrin as a therapeutic target could result in breakthroughs for the treatment of neurodegenerative diseases and brain injury in mammals.
Keywords: CNS; astrocyte; axon; invertebrate; mammal; mouse; neuron; phylogenetic tree; regeneration; teleost fish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Use of Cre-lox technology to analyze integrin functions in astrocytes.Methods Mol Biol. 2012;814:555-70. doi: 10.1007/978-1-61779-452-0_37. Methods Mol Biol. 2012. PMID: 22144332 Review.
-
Extracellular matrix composition determines astrocyte responses to mechanical and inflammatory stimuli.Neurosci Lett. 2015 Jul 23;600:104-9. doi: 10.1016/j.neulet.2015.06.013. Epub 2015 Jun 9. Neurosci Lett. 2015. PMID: 26067407 Free PMC article.
-
Astrocyte-secreted matricellular proteins in CNS remodelling during development and disease.Neural Plast. 2014;2014:321209. doi: 10.1155/2014/321209. Epub 2014 Jan 16. Neural Plast. 2014. PMID: 24551460 Free PMC article. Review.
-
Astrocytoma adhesion to extracellular matrix: functional significance of integrin and focal adhesion kinase expression.J Neuropathol Exp Neurol. 1999 Feb;58(2):198-209. doi: 10.1097/00005072-199902000-00009. J Neuropathol Exp Neurol. 1999. PMID: 10029102
-
Increased expression of the beta4 and alpha5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system.Mol Cell Neurosci. 2006 Dec;33(4):429-40. doi: 10.1016/j.mcn.2006.09.004. Epub 2006 Oct 13. Mol Cell Neurosci. 2006. PMID: 17049262 Free PMC article.
Cited by
-
Recapitulation of pathophysiological features of AD in SARS-CoV-2-infected subjects.Elife. 2023 Jul 7;12:e86333. doi: 10.7554/eLife.86333. Elife. 2023. PMID: 37417740 Free PMC article.
-
Hindbrain boundaries as niches of neural progenitor and stem cells regulated by the extracellular matrix proteoglycan chondroitin sulphate.Development. 2024 Feb 15;151(4):dev201934. doi: 10.1242/dev.201934. Epub 2024 Feb 13. Development. 2024. PMID: 38251863 Free PMC article.
-
The Serine/Threonine Kinase NDR2 Regulates Integrin Signaling, Synapse Formation, and Synaptic Plasticity in the Hippocampus.J Neurochem. 2025 Jun;169(6):e70094. doi: 10.1111/jnc.70094. J Neurochem. 2025. PMID: 40439020 Free PMC article.
-
Abnormalities in Copper Status Associated with an Elevated Risk of Parkinson's Phenotype Development.Antioxidants (Basel). 2023 Aug 22;12(9):1654. doi: 10.3390/antiox12091654. Antioxidants (Basel). 2023. PMID: 37759957 Free PMC article. Review.
-
Water-Soluble Lynx1 Upregulates Dendritic Spine Density and Stimulates Astrocytic Network and Signaling.Mol Neurobiol. 2025 May;62(5):5531-5545. doi: 10.1007/s12035-024-04627-1. Epub 2024 Nov 20. Mol Neurobiol. 2025. PMID: 39565568
References
-
- Harrison R.G. Observations on the livining developing nerve fiber. Proc. Soc. Exp. Biol. Med. 1907;4:140–143. doi: 10.3181/00379727-4-98. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

