Assays of CFTR Function In Vitro, Ex Vivo and In Vivo
- PMID: 35163362
- PMCID: PMC8836180
- DOI: 10.3390/ijms23031437
Assays of CFTR Function In Vitro, Ex Vivo and In Vivo
Abstract
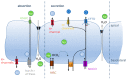
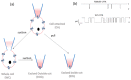
Cystic fibrosis, a multi-organ genetic disease, is characterized by abnormal function of the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a chloride channel at the apical membrane of several epithelia. In recent years, therapeutic strategies have been developed to correct the CFTR defect. To evaluate CFTR function at baseline for diagnosis, or the efficacy of CFTR-restoring therapy, reliable tests are needed to measure CFTR function, in vitro, ex vivo and in vivo. In vitro techniques either directly or indirectly measure ion fluxes; direct measurement of ion fluxes and quenching of fluorescence in cell-based assays, change in transmembrane voltage or current in patch clamp or Ussing chamber, swelling of CFTR-containing organoids by secondary water influx upon CFTR activation. Several cell or tissue types can be used. Ex vivo and in vivo assays similarly evaluate current (intestinal current measurement) and membrane potential differences (nasal potential difference), on tissues from individual patients. In the sweat test, the most frequently used in vivo evaluation of CFTR function, chloride concentration or stimulated sweat rate can be directly measured. Here, we will describe the currently available bio-assays for quantitative evaluation of CFTR function, their indications, advantages and disadvantages, and correlation with clinical outcome measures.
Keywords: CFTR function; anion channel; bioassay; biomarker; cystic fibrosis.
Conflict of interest statement
The authors declare no conflict of interest related to this paper.
Figures
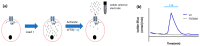

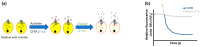




Similar articles
-
Correlating genotype with phenotype using CFTR-mediated whole-cell Cl- currents in human nasal epithelial cells.J Physiol. 2022 Mar;600(6):1515-1531. doi: 10.1113/JP282143. Epub 2021 Dec 8. J Physiol. 2022. PMID: 34761808
-
Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue.Am J Respir Cell Mol Biol. 2010 Nov;43(5):607-16. doi: 10.1165/rcmb.2009-0281OC. Epub 2009 Dec 30. Am J Respir Cell Mol Biol. 2010. PMID: 20042712 Free PMC article.
-
Effect of apical chloride concentration on the measurement of responses to CFTR modulation in airway epithelia cultured from nasal brushings.Physiol Rep. 2020 Oct;8(19):e14603. doi: 10.14814/phy2.14603. Physiol Rep. 2020. PMID: 33038073 Free PMC article.
-
Diagnostic tools and CFTR functional assays in cystic fibrosis: utility and availability in Switzerland.Swiss Med Wkly. 2021 Apr 13;151:w20496. doi: 10.4414/smw.2021.20496. eCollection 2021 Apr 12. Swiss Med Wkly. 2021. PMID: 33934316 Review.
-
Ex vivo CF diagnosis by intestinal current measurements (ICM) in small aperture, circulating Ussing chambers.J Cyst Fibros. 2004 Aug;3 Suppl 2:159-63. doi: 10.1016/j.jcf.2004.05.034. J Cyst Fibros. 2004. PMID: 15463951 Review.
Cited by
-
Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders.Antioxidants (Basel). 2022 Mar 15;11(3):555. doi: 10.3390/antiox11030555. Antioxidants (Basel). 2022. PMID: 35326205 Free PMC article. Review.
-
On the path to predicting immune responses in the lung: Modeling the pulmonary innate immune system at the air-liquid interface (ALI).Eur J Pharm Sci. 2023 Dec 1;191:106596. doi: 10.1016/j.ejps.2023.106596. Epub 2023 Sep 26. Eur J Pharm Sci. 2023. PMID: 37770004 Free PMC article. Review.
-
Differential expression of ion channel coding genes in the endometrium of women experiencing recurrent implantation failures.Sci Rep. 2024 Aug 27;14(1):19822. doi: 10.1038/s41598-024-70778-9. Sci Rep. 2024. PMID: 39192025 Free PMC article.
-
Adenine base editing with engineered virus-like particles rescues the CFTR mutation G542X in patient-derived intestinal organoids.iScience. 2025 Feb 21;28(3):111979. doi: 10.1016/j.isci.2025.111979. eCollection 2025 Mar 21. iScience. 2025. PMID: 40144632 Free PMC article.
-
CRISPR/Cas9 bioluminescence-based assay for monitoring CFTR trafficking to the plasma membrane.Life Sci Alliance. 2023 Nov 2;7(1):e202302045. doi: 10.26508/lsa.202302045. Print 2024 Jan. Life Sci Alliance. 2023. PMID: 37918963 Free PMC article.
References
-
- Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E., Mall M.A., Welter J.J., Ramsey B.W., McKee C.M., et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet. 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. - DOI - PMC - PubMed
-
- Middleton P.G., Mall M.A., Dřevínek P., Lands L.C., McKone E.F., Polineni D., Ramsey B.W., Taylor-Cousar J.L., Tullis E., Vermeulen F., et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. - DOI - PMC - PubMed
-
- Who Is TRIKAFTA® For?|TRIKAFTA® (Elexacaftor/Tezacaftor/Ivacaftor and Ivacaftor) n.d. [(accessed on 6 January 2022)]. Available online: https://www.trikafta.com/who-trikafta-is-for.
Publication types
MeSH terms
Substances
Grants and funding
- 12Z5920N/Research Foundation - Flanders
- S001221N/Research Foundation - Flanders
- 2020-J1810150-217926/Belgian CF patient Association and the Fund Alphonse Jean Forton from the King Baudouin Foundation
- 2020-J1810150-E015/Belgian CF patient Association and the Fund Alphonse Jean Forton from the King Baudouin Foundation
- 2020-J1810150-217924/Belgian CF patient Association and the Fund Alphonse Jean Forton from the King Baudouin Foundation
LinkOut - more resources
Full Text Sources
Medical

