Transcriptomic Profile of Canine DH82 Macrophages Infected by Leishmania infantum Promastigotes with Different Virulence Behavior
- PMID: 35163386
- PMCID: PMC8835757
- DOI: 10.3390/ijms23031466
Transcriptomic Profile of Canine DH82 Macrophages Infected by Leishmania infantum Promastigotes with Different Virulence Behavior
Abstract
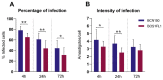
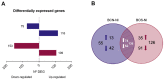
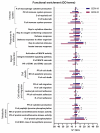
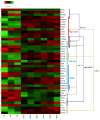
Zoonotic visceral leishmaniosis caused by Leishmania infantum is an endemic disease in the Mediterranean Basin affecting mainly humans and dogs, the main reservoir. The leishmaniosis outbreak declared in the Community of Madrid (Spain) led to a significant increase in human disease incidence without enhancing canine leishmaniosis prevalence, suggesting a better adaptation of the outbreak's isolates by other host species. One of the isolates obtained in the focus, IPER/ES/2012/BOS1FL1 (BOS1FL1), has previously demonstrated a different phenotype than the reference strain MCAN/ES/1996/BCN150 (BCN150), characterized by a lower infectivity when interacting with canine macrophages. Nevertheless, not enough changes in the cell defensive response were found to support their different behavior. Thus, we decided to investigate the molecular mechanisms involved in the interaction of both parasites with DH82 canine macrophages by studying their transcriptomic profiles developed after infection using RNA sequencing. The results showed a common regulation induced by both parasites in the phosphoinositide-3-kinase-protein kinase B/Akt and NOD-like receptor signaling pathways. However, other pathways, such as phagocytosis and signal transduction, including tumor necrosis factor, mitogen-activated kinases and nuclear factor-κB, were only regulated after infection with BOS1FL1. These differences could contribute to the reduced infection ability of the outbreak isolates in canine cells. Our results open a new avenue to investigate the true role of adaptation of L. infantum isolates in their interaction with their different hosts.
Keywords: DH82 cells; Leishmania infantum; RNA-seq; canine macrophage; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Carrillo E., Crusat M., Nieto J., Chicharro C., Thomas M.D.C., Martínez E., Valladares B., Cañavate C., Requena J.M., López M.C., et al. Immunogenicity of HSP-70, KMP-11 and PFR-2 leishmanial antigens in the experimental model of canine visceral leishmaniasis. Vaccine. 2008;26:1902–1911. doi: 10.1016/j.vaccine.2008.01.042. - DOI - PubMed
-
- Horrillo L., Castro A., Matía B., Molina L., García-Martínez J., Jaqueti J., García-Arata I., Carrillo E., Moreno J., Ruiz-Giardin J.M., et al. Clinical aspects of visceral leishmaniasis caused by L. infantum in adults. Ten years of experience of the largest outbreak in Europe: What have we learned? Parasites Vectors. 2019;12:359. doi: 10.1186/s13071-019-3628-z. - DOI - PMC - PubMed
-
- Arce A., Estirado A., Ordobas M., Sevilla S., García N., Moratilla L., De La Fuente S., Martínez A.M., Pérez A.M., Aránguez E., et al. Re-emergence of leishmaniasis in Spain: Community outbreak in Madrid, Spain, 2009 to 2012. Eurosurveillance. 2013;18:20546. doi: 10.2807/1560-7917.ES2013.18.30.20546. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

