Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression
- PMID: 35163468
- PMCID: PMC8836259
- DOI: 10.3390/ijms23031548
Oncogenic Mutation BRAF V600E Changes Phenotypic Behavior of THLE-2 Liver Cells through Alteration of Gene Expression
Abstract
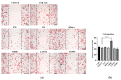
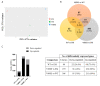
The accumulation of mutations in cancer driver genes, such as tumor suppressors or proto-oncogenes, affects cellular homeostasis. Disturbances in the mechanism controlling proliferation cause significant augmentation of cell growth and division due to the loss of sensitivity to the regulatory signals. Nowadays, an increasing number of cases of liver cancer are observed worldwide. Data provided by the International Cancer Genome Consortium (ICGC) have indicated many alterations within gene sequences, whose roles in tumor development are not well understood. A comprehensive analysis of liver cancer (virus-associated hepatocellular carcinoma) samples has identified new and rare mutations in B-Raf proto-oncogene (BRAF) in Japanese HCC patients, as well as BRAF V600E mutations in French HCC patients. However, their function in liver cancer has never been investigated. Here, using functional analysis and next generation sequencing, we demonstrate the tumorigenic effect of BRAF V600E on hepatocytes (THLE-2 cell line). Moreover, we identified genes such as BMP6, CXCL11, IL1B, TBX21, RSAD2, MMP10, and SERPIND1, which are possibly regulated by the BRAF V600E-mediated, mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) signaling pathway. Through several functional assays, we demonstrate that BRAF L537M, D594A, and E648G mutations alone are not pathogenic in liver cancer. The investigation of genome mutations and the determination of their impact on cellular processes and functions is crucial to unraveling the molecular mechanisms of liver cancer development.
Keywords: BRAF mutation; MAPK/ERK; hepatocellular carcinoma; liver cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

