Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production
- PMID: 35163474
- PMCID: PMC8836166
- DOI: 10.3390/ijms23031530
Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production
Abstract
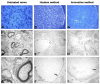
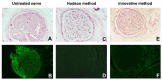
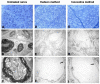
Defects of the peripheral nervous system are extremely frequent in trauma and surgeries and have high socioeconomic costs. If the direct suture of a lesion is not possible, i.e., nerve gap > 2 cm, it is necessary to use grafts. While the gold standard is the autograft, it has disadvantages related to its harvesting, with an inevitable functional deficit and further morbidity. An alternative to autografting is represented by the acellular nerve allograft (ANA), which avoids disadvantages of autograft harvesting and fresh allograft rejection. In this research, the authors intend to transfer to human nerves a novel technique, previously implemented in animal models, to decellularize nerves. The new method is based on soaking the nerve tissues in decellularizing solutions while associating ultrasounds and freeze-thaw cycles. It is performed without interrupting the sterility chain, so that the new graft may not require post-production γ-ray irradiation, which is suspected to affect the structural and functional quality of tissues. The new method is rapid, safe, and inexpensive if compared with available commercial ANAs. Histology and immunohistochemistry have been adopted to evaluate the new decellularized nerves. The study shows that the new method can be applied to human nerve samples, obtaining similar, and, sometimes better, results compared with the chosen control method, the Hudson technique.
Keywords: allografting; decellularization; nerve regeneration; orthopedic and maxillofacial surgery; reconstructive surgery; tissue transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- López-Cebral R., Silva-Correia J., Reis R.L., Silva T.H., Oliveira J.M. Peripheral nerve injury: Current challenges, conventional treatment approaches, and new trends in biomaterials-based regenerative strategies. ACS Biomater. Sci. Eng. 2017;3:3098–3122. doi: 10.1021/acsbiomaterials.7b00655. - DOI - PubMed
-
- Huckhagel T., Nüchtern J., Regelsberger J., Lefering R. Nerve injury in severe trauma with upper extremity involvement: Evaluation of 49,382 patients from the TraumaRegister DGU® between 2002 and 2015. Scand. J. Trauma Resusc. Emerg. Med. 2018;26:76. doi: 10.1186/s13049-018-0546-6. - DOI - PMC - PubMed
-
- Fogagnolo P., Giannaccare G., Bolognesi F., Digiuni M., Tranchina L., Rossetti L., Dipinto A., Allevi F., Lozza A., Rabbiosi D., et al. Direct versus indirect corneal neurotization for the treatment of neurotrophic keratopathy: A multicenter prospective comparative study. Am. J. Ophthalmol. 2020;220:203–214. doi: 10.1016/j.ajo.2020.07.003. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

