Polymodal Control of TMEM16x Channels and Scramblases
- PMID: 35163502
- PMCID: PMC8835819
- DOI: 10.3390/ijms23031580
Polymodal Control of TMEM16x Channels and Scramblases
Abstract
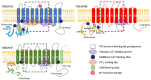
The TMEM16A/anoctamin-1 calcium-activated chloride channel (CaCC) contributes to a range of vital functions, such as the control of vascular tone and epithelial ion transport. The channel is a founding member of a family of 10 proteins (TMEM16x) with varied functions; some members (i.e., TMEM16A and TMEM16B) serve as CaCCs, while others are lipid scramblases, combine channel and scramblase function, or perform additional cellular roles. TMEM16x proteins are typically activated by agonist-induced Ca2+ release evoked by Gq-protein-coupled receptor (GqPCR) activation; thus, TMEM16x proteins link Ca2+-signalling with cell electrical activity and/or lipid transport. Recent studies demonstrate that a range of other cellular factors-including plasmalemmal lipids, pH, hypoxia, ATP and auxiliary proteins-also control the activity of the TMEM16A channel and its paralogues, suggesting that the TMEM16x proteins are effectively polymodal sensors of cellular homeostasis. Here, we review the molecular pathophysiology, structural biology, and mechanisms of regulation of TMEM16x proteins by multiple cellular factors.
Keywords: Ca2+ signalling; Ca2+-activated Cl− channels; SARS-CoV-2; TMEM16x; anoctamin; gating; lipids; scramblases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bushell S.R., Pike A.C.W., Falzone M.E., Rorsman N.J.G., Ta C.M., Corey R.A., Newport T.D., Christianson J.C., Scofano L.F., Shintre C.A., et al. The Structural Basis of Lipid Scrambling and Inactivation in the Endoplasmic Reticulum Scramblase TMEM16K. Nat. Commun. 2019;10:3956. doi: 10.1038/s41467-019-11753-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

