Implantable Immunosuppressant Delivery to Prevent Rejection in Transplantation
- PMID: 35163514
- PMCID: PMC8835747
- DOI: 10.3390/ijms23031592
Implantable Immunosuppressant Delivery to Prevent Rejection in Transplantation
Abstract
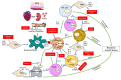
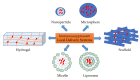
An innovative immunosuppressant with a minimally invasive delivery system has emerged in the biomedical field. The application of biodegradable and biocompatible polymer forms, such as hydrogels, scaffolds, microspheres, and nanoparticles, in transplant recipients to control the release of immunosuppressants can minimize the risk of developing unfavorable conditions. In this review, we summarized several studies that have used implantable immunosuppressant delivery to release therapeutic agents to prolong allograft survival. We also compared their applications, efficacy, efficiency, and safety/side effects with conventional therapeutic-agent administration. Finally, challenges and the future prospective were discussed. Collectively, this review will help relevant readers understand the different approaches to prevent transplant rejection in a new era of therapeutic agent delivery.
Keywords: immunosuppressant; implantable drug delivery; rejection; transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Design and Optimization of PLGA Particles to Deliver Immunomodulatory Drugs for the Prevention of Skin Allograft Rejection.Immunol Invest. 2020 Oct;49(7):840-857. doi: 10.1080/08820139.2019.1695134. Epub 2019 Dec 6. Immunol Invest. 2020. PMID: 31809611
-
Nanoparticle-assisted Targeting Delivery Technologies for Preventing Organ Rejection.Transplantation. 2024 Nov 1;108(11):2174-2185. doi: 10.1097/TP.0000000000005025. Epub 2024 Apr 8. Transplantation. 2024. PMID: 38597913 Review.
-
Application of PLGA nano/microparticle delivery systems for immunomodulation and prevention of allotransplant rejection.Expert Opin Drug Deliv. 2020 Jun;17(6):767-780. doi: 10.1080/17425247.2020.1748006. Epub 2020 Apr 4. Expert Opin Drug Deliv. 2020. PMID: 32223341 Review.
-
Micro and nanoparticle drug delivery systems for preventing allotransplant rejection.Clin Immunol. 2015 Sep;160(1):24-35. doi: 10.1016/j.clim.2015.04.013. Epub 2015 May 1. Clin Immunol. 2015. PMID: 25937032 Free PMC article. Review.
-
Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option?Paediatr Drugs. 2011 Feb 1;13(1):49-69. doi: 10.2165/11538530-000000000-00000. Paediatr Drugs. 2011. PMID: 21162600 Review.
Cited by
-
Immunoprotection of cellular transplants for autoimmune type 1 diabetes through local drug delivery.Adv Drug Deliv Rev. 2024 Mar;206:115179. doi: 10.1016/j.addr.2024.115179. Epub 2024 Jan 28. Adv Drug Deliv Rev. 2024. PMID: 38286164 Free PMC article. Review.
-
Dual-Drug Delivery Systems Using Hydrogel-Nanoparticle Composites: Recent Advances and Key Applications.Gels. 2025 Jul 3;11(7):520. doi: 10.3390/gels11070520. Gels. 2025. PMID: 40710682 Free PMC article. Review.
-
Nanomedicine and nanoparticle-based delivery systems in plastic and reconstructive surgery.Maxillofac Plast Reconstr Surg. 2023 Mar 30;45(1):15. doi: 10.1186/s40902-023-00383-9. Maxillofac Plast Reconstr Surg. 2023. PMID: 36995508 Free PMC article. Review.
References
-
- Flaherty D.K. Transplantation. Elsevier; St. Louis, MO, USA: 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

