The Enhanced Cytotoxic Effects in B-Cell Leukemia and Lymphoma Following Activation of Prostaglandin EP4 Receptor and Targeting of CD20 Antigen by Monoclonal Antibodies
- PMID: 35163524
- PMCID: PMC8835876
- DOI: 10.3390/ijms23031599
The Enhanced Cytotoxic Effects in B-Cell Leukemia and Lymphoma Following Activation of Prostaglandin EP4 Receptor and Targeting of CD20 Antigen by Monoclonal Antibodies
Abstract
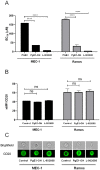
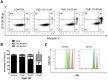
Anti-CD20 monoclonal antibodies (MAbs) have revolutionized the treatment of B-cell leukemia and lymphoma. However, many patients do not respond to such treatment due to either deficiency of the complementary immune response or resistance to apoptosis. Other currently available treatments are often inadequate or induce major side effects. Therefore, there is a constant need for improved therapies. The prostaglandin E2 receptor 4 (EP4) receptor has been identified as a promising therapeutic target for hematologic B-cell malignancies. Herein, we report that EP4 receptor agonists PgE1-OH and L-902688 have exhibited enhanced cytotoxicity when applied together with anti-CD20 MAbs rituximab, ofatumumab and obinutuzumab in vitro in Burkitt lymphoma cells Ramos, as well as in p53-deficient chronic lymphocytic leukemia (CLL) cells MEC-1. Moreover, the enhanced cytotoxic effects of EP4 receptor agonists and MAbs targeting CD20 have been identified ex vivo on primary lymphocytes B obtained from patients diagnosed with CLL. Incubation of cells with PgE1-OH and L-902688 preserved the expression of CD20 molecules, further confirming the anti-leukemic potential of EP4 receptor agonists in combination with anti-CD20 MAbs. Additionally, we demonstrated that the EP4 receptor agonist PgE-1-OH induced apoptosis and inhibited proliferation via the EP4 receptor triggering in CLL. This work has revealed very important findings leading towards the elucidation of the anticancer potential of PgE1-OH and L-902688, either alone or in combination with MAbs. This may contribute to the development of potential therapeutic alternatives for patients with B-cell malignancies.
Keywords: B-cell leukemia and lymphoma; chronic lymphocytic leukemia; monoclonal antibodies; prostaglandin EP4 receptor; selective EP4 receptor agonist; synergistic effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

