New Evidence of the Importance of Weak Interactions in the Formation of PML-Bodies
- PMID: 35163537
- PMCID: PMC8835755
- DOI: 10.3390/ijms23031613
New Evidence of the Importance of Weak Interactions in the Formation of PML-Bodies
Abstract
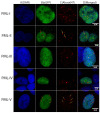
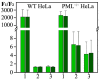
In this work, we performed a comparative study of the formation of PML bodies by full-length PML isoforms and their C-terminal domains in the presence and absence of endogenous PML. Based on the analysis of the distribution of intrinsic disorder predisposition in the amino acid sequences of PML isoforms, regions starting from the amino acid residue 395 (i.e., sequences encoded by exons 4-6) were assigned as the C-terminal domains of these proteins. We demonstrate that each of the full-sized nuclear isoforms of PML is capable of forming nuclear liquid-droplet compartments in the absence of other PML isoforms. These droplets possess dynamic characteristics of the exchange with the nucleoplasm close to those observed in the wild-type cells. Only the C-terminal domains of the PML-II and PML-V isoforms are able to be included in the composition of the endogenous PML bodies, while being partially distributed in the nucleoplasm. The bodies formed by the C-terminal domain of the PML-II isoform are dynamic liquid droplet compartments, regardless of the presence or absence of endogenous PML. The C-terminal domain of PML-V forms dynamic liquid droplet compartments in the knockout cells (PML-/-), but when the C-terminus of the PML-V isoform is inserted into the existing endogenous PML bodies, the molecules of this protein cease to exchange with the nucleoplasm. It was demonstrated that the K490R substitution, which disrupts the PML sumoylation, promotes diffuse distribution of the C-terminal domains of PML-II and PML-V isoforms in endogenous PML knockout HeLa cells, but not in the wild-type cells. These data indicate the ability of the C-terminal domains of the PML-II and PML-V isoforms to form dynamic liquid droplet-like compartments, regardless of the ordered N-terminal RBCC motifs of the PML. This indicates a significant role of the non-specific interactions between the mostly disordered C-terminal domains of PML isoforms for the initiation of liquid-liquid phase separation (LLPS) leading to the formation of PML bodies.
Keywords: PML-bodies; acute hydrogen peroxide-induced oxidative stress; fluorescence recovery after photobleaching (FRAP); liquid–liquid phase separation (LLPS); membrane-less organelles (MLOs); promyelocytic leukemia protein (PML) isoforms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

