State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis
- PMID: 35163541
- PMCID: PMC8835711
- DOI: 10.3390/ijms23031618
State of the Art: The Immunomodulatory Role of MSCs for Osteoarthritis
Abstract
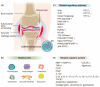
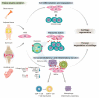
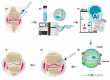
Osteoarthritis (OA) has generally been introduced as a degenerative disease; however, it has recently been understood as a low-grade chronic inflammatory process that could promote symptoms and accelerate the progression of OA. Current treatment strategies, including corticosteroid injections, have no impact on the OA disease progression. Mesenchymal stem cells (MSCs) based therapy seem to be in the spotlight as a disease-modifying treatment because this strategy provides enlarged anti-inflammatory and chondroprotective effects. Currently, bone marrow, adipose derived, synovium-derived, and Wharton's jelly-derived MSCs are the most widely used types of MSCs in the cartilage engineering. MSCs exert immunomodulatory, immunosuppressive, antiapoptotic, and chondrogenic effects mainly by paracrine effect. Because MSCs disappear from the tissue quickly after administration, recently, MSCs-derived exosomes received the focus for the next-generation treatment strategy for OA. MSCs-derived exosomes contain a variety of miRNAs. Exosomal miRNAs have a critical role in cartilage regeneration by immunomodulatory function such as promoting chondrocyte proliferation, matrix secretion, and subsiding inflammation. In the future, a personalized exosome can be packaged with ideal miRNA and proteins for chondrogenesis by enriching techniques. In addition, the target specific exosomes could be a gamechanger for OA. However, we should consider the off-target side effects due to multiple gene targets of miRNA.
Keywords: antiinflammation; exosome; immunomodulation; mesenchymal stem cell; miRNA; osteoarthritis.
Conflict of interest statement
The authors have no conflict of interest.
Figures
References
-
- He L., He T., Xing J., Zhou Q., Fan L., Liu C., Chen Y., Wu D., Tian Z., Liu B., et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020;11:1–15. doi: 10.1186/s13287-020-01781-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

