Development of Planar Illumination Strategies for Solving Mysteries in the Sub-Cellular Realm
- PMID: 35163562
- PMCID: PMC8835835
- DOI: 10.3390/ijms23031643
Development of Planar Illumination Strategies for Solving Mysteries in the Sub-Cellular Realm
Abstract
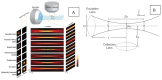
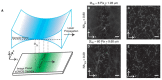
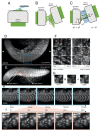
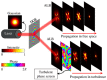
Optical microscopy has vastly expanded the frontiers of structural and functional biology, due to the non-invasive probing of dynamic volumes in vivo. However, traditional widefield microscopy illuminating the entire field of view (FOV) is adversely affected by out-of-focus light scatter. Consequently, standard upright or inverted microscopes are inept in sampling diffraction-limited volumes smaller than the optical system's point spread function (PSF). Over the last few decades, several planar and structured (sinusoidal) illumination modalities have offered unprecedented access to sub-cellular organelles and 4D (3D + time) image acquisition. Furthermore, these optical sectioning systems remain unaffected by the size of biological samples, providing high signal-to-noise (SNR) ratios for objective lenses (OLs) with long working distances (WDs). This review aims to guide biologists regarding planar illumination strategies, capable of harnessing sub-micron spatial resolution with a millimeter depth of penetration.
Keywords: axially swept light sheet; lattice light sheet; light sheet microscope; oblique plane illumination; single-molecule localization light sheet; sub-voxel resolving technique; super-resolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
3D single-molecule super-resolution microscopy with a tilted light sheet.Nat Commun. 2018 Jan 9;9(1):123. doi: 10.1038/s41467-017-02563-4. Nat Commun. 2018. PMID: 29317629 Free PMC article.
-
Deconvolution-free Subcellular Imaging with Axially Swept Light Sheet Microscopy.Biophys J. 2015 Jun 16;108(12):2807-15. doi: 10.1016/j.bpj.2015.05.013. Biophys J. 2015. PMID: 26083920 Free PMC article.
-
Resolution doubling in light-sheet microscopy via oblique plane structured illumination.Nat Methods. 2022 Nov;19(11):1419-1426. doi: 10.1038/s41592-022-01635-8. Epub 2022 Oct 24. Nat Methods. 2022. PMID: 36280718 Free PMC article.
-
Light sheet approaches for improved precision in 3D localization-based super-resolution imaging in mammalian cells [Invited].Opt Express. 2018 May 14;26(10):13122-13147. doi: 10.1364/OE.26.013122. Opt Express. 2018. PMID: 29801343 Free PMC article. Review.
-
Optical sectioning microscopy with planar or structured illumination.Nat Methods. 2011 Sep 29;8(10):811-9. doi: 10.1038/nmeth.1709. Nat Methods. 2011. PMID: 21959136 Review.
Cited by
-
Scale space detector for analyzing spatiotemporal ventricular contractility and nuclear morphogenesis in zebrafish.iScience. 2022 Aug 5;25(9):104876. doi: 10.1016/j.isci.2022.104876. eCollection 2022 Sep 16. iScience. 2022. PMID: 36034231 Free PMC article.
-
Toxicity and assimilation of cellulosic copper nanoparticles require α-arrestins in S. cerevisiae.Metallomics. 2023 Mar 6;15(3):mfad011. doi: 10.1093/mtomcs/mfad011. Metallomics. 2023. PMID: 36841230 Free PMC article.
References
-
- Figueroa B., Xu F.X., Hu R., Men S., Fu D. Quantitative Imaging of Intracellular Density with Ratiometric Stimulated Raman Scattering Microscopy. bioRxiv. 2021 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

