Upregulation of miR-34a-5p, miR-20a-3p and miR-29a-3p by Onconase in A375 Melanoma Cells Correlates with the Downregulation of Specific Onco-Proteins
- PMID: 35163570
- PMCID: PMC8835754
- DOI: 10.3390/ijms23031647
Upregulation of miR-34a-5p, miR-20a-3p and miR-29a-3p by Onconase in A375 Melanoma Cells Correlates with the Downregulation of Specific Onco-Proteins
Abstract
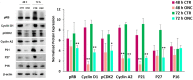
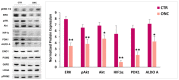
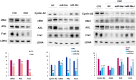
Onconase (ONC) is an amphibian secretory ribonuclease displaying cytostatic and cytotoxic activities against many mammalian tumors, including melanoma. ONC principally damages tRNA species, but also other non-coding RNAs, although its precise targets are not known. We investigated the ONC ability to modulate the expression of 16 onco-suppressor microRNAs (miRNAs) in the A375 BRAF-mutated melanoma cell line. RT-PCR and immunoblots were used to measure the expression levels of miRNAs and their regulated proteins, respectively. In silico study was carried out to verify the relations between miRNAs and their mRNA targets. A375 cell transfection with miR-20a-3p and miR-34a-5p mimics or inhibitors was performed. The onco-suppressors miR-20a-3p, miR-29a-3p and miR-34a-5p were highly expressed in 48-h ONC-treated A375 cells. The cytostatic effect of ONC in A375 cells was mechanistically explained by the sharp inhibition of cyclins D1 and A2 expression level, as well as by downregulation of retinoblastoma protein and cyclin-dependent-kinase-2 activities. Remarkably, the expression of kinases ERK1/2 and Akt, as well as of the hypoxia inducible factor-1α, was inhibited by ONC. All these proteins control pro-survival pathways. Finally, many crucial proteins involved in migration, invasion and metastatic potential were downregulated by ONC. Results obtained from transfection of miR-20a-3p and miR-34a-5p inhibitors in the presence of ONC show that these miRNAs may participate in the antitumor effects of ONC in the A375 cell line. In conclusion, we identified many intracellular downregulated proteins involved in melanoma cell proliferation, metabolism and progression. All mRNAs coding these proteins may be targets of miR-20a-3p, miR-29a-3p and/or miR-34a-5p, which are in turn upregulated by ONC. Data suggest that several known ONC anti-proliferative and anti-metastatic activities in A375 melanoma cells might depend on the upregulation of onco-suppressor miRNAs. Notably, miRNAs stability depends on the upstream regulation by long-non-coding-RNAs or circular-RNAs that can, in turn, be damaged by ONC ribonucleolytic activity.
Keywords: AXL; CREB; Fra1; HIF1α; PDK1; SIRT1; SOX2; cMet; cyclin A2; cyclin D1; ribonuclease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Role of the Ribonuclease ONCONASE in miRNA Biogenesis and tRNA Processing: Focus on Cancer and Viral Infections.Int J Mol Sci. 2022 Jun 12;23(12):6556. doi: 10.3390/ijms23126556. Int J Mol Sci. 2022. PMID: 35742999 Free PMC article. Review.
-
Circular RNA circ_0001591 Contributes to Melanoma Cell Migration Through AXL and FRA1 Proteins by Targeting miR-20a-3p and miR-34a-5p.Genes (Basel). 2025 Jul 30;16(8):921. doi: 10.3390/genes16080921. Genes (Basel). 2025. PMID: 40869968 Free PMC article.
-
Influence of onconase in the therapeutic potential of PARP inhibitors in A375 malignant melanoma cells.Biochem Pharmacol. 2019 Sep;167:173-181. doi: 10.1016/j.bcp.2019.06.006. Epub 2019 Jun 8. Biochem Pharmacol. 2019. PMID: 31185226
-
Tumor Suppressor Role of hsa-miR-193a-3p and -5p in Cutaneous Melanoma.Int J Mol Sci. 2020 Aug 27;21(17):6183. doi: 10.3390/ijms21176183. Int J Mol Sci. 2020. PMID: 32867069 Free PMC article.
-
Dysregulated expression and functions of microRNA-330 in cancers: A potential therapeutic target.Biomed Pharmacother. 2022 Feb;146:112600. doi: 10.1016/j.biopha.2021.112600. Epub 2021 Dec 27. Biomed Pharmacother. 2022. PMID: 34968919
Cited by
-
Role of the Ribonuclease ONCONASE in miRNA Biogenesis and tRNA Processing: Focus on Cancer and Viral Infections.Int J Mol Sci. 2022 Jun 12;23(12):6556. doi: 10.3390/ijms23126556. Int J Mol Sci. 2022. PMID: 35742999 Free PMC article. Review.
-
Evaluation of the Anti-Cancer Potential of Extracellular Vesicles Derived from Human Amniotic Fluid Stem Cells: Focus on Effective miRNAs in the Treatment of Melanoma Progression.Int J Mol Sci. 2024 Nov 21;25(23):12502. doi: 10.3390/ijms252312502. Int J Mol Sci. 2024. PMID: 39684214 Free PMC article.
-
Noncoding RNAs as regulators of FOSL1 in cancer.Front Immunol. 2025 Aug 1;16:1599674. doi: 10.3389/fimmu.2025.1599674. eCollection 2025. Front Immunol. 2025. PMID: 40821785 Free PMC article. Review.
-
Identification of expression profiles and prognostic value of RFCs in colorectal cancer.Sci Rep. 2024 Mar 19;14(1):6607. doi: 10.1038/s41598-024-56361-2. Sci Rep. 2024. PMID: 38504096 Free PMC article.
-
The Role of Non-coding RNAs in Tumorigenesis, Diagnosis/Prognosis, and Therapeutic Strategies for Cutaneous Melanoma.Methods Mol Biol. 2025;2883:79-107. doi: 10.1007/978-1-0716-4290-0_4. Methods Mol Biol. 2025. PMID: 39702705 Review.
References
-
- Lee I., Kalota A., Gewirtz A.M., Shogen K. Antitumor Efficacy of the Cytotoxic RNase, Ranpirnase, on A549 Human Lung Cancer Xenografts of Nude Mice. Anticancer Res. 2007;27:299–307. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

