Broad Specific Xyloglucan:Xyloglucosyl Transferases Are Formidable Players in the Re-Modelling of Plant Cell Wall Structures
- PMID: 35163576
- PMCID: PMC8836008
- DOI: 10.3390/ijms23031656
Broad Specific Xyloglucan:Xyloglucosyl Transferases Are Formidable Players in the Re-Modelling of Plant Cell Wall Structures
Abstract
Plant xyloglucan:xyloglucosyl transferases, known as xyloglucan endo-transglycosylases (XETs) are the key players that underlie plant cell wall dynamics and mechanics. These fundamental roles are central for the assembly and modifications of cell walls during embryogenesis, vegetative and reproductive growth, and adaptations to living environments under biotic and abiotic (environmental) stresses. XET enzymes (EC 2.4.1.207) have the β-sandwich architecture and the β-jelly-roll topology, and are classified in the glycoside hydrolase family 16 based on their evolutionary history. XET enzymes catalyse transglycosylation reactions with xyloglucan (XG)-derived and other than XG-derived donors and acceptors, and this poly-specificity originates from the structural plasticity and evolutionary diversification that has evolved through expansion and duplication. In phyletic groups, XETs form the gene families that are differentially expressed in organs and tissues in time- and space-dependent manners, and in response to environmental conditions. Here, we examine higher plant XET enzymes and dissect how their exclusively carbohydrate-linked transglycosylation catalytic function inter-connects complex plant cell wall components. Further, we discuss progress in technologies that advance the knowledge of plant cell walls and how this knowledge defines the roles of XETs. We construe that the broad specificity of the plant XETs underscores their roles in continuous cell wall restructuring and re-modelling.
Keywords: crystal structures; evolutionary history; glycoside hydrolase family 16; mechanism of catalysis; molecular modelling and dynamics; substrate binding; transglycosylation reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
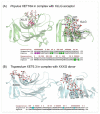



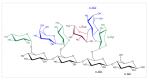
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

