Nitric Oxide Signaling and Its Association with Ubiquitin-Mediated Proteasomal Degradation in Plants
- PMID: 35163578
- PMCID: PMC8835921
- DOI: 10.3390/ijms23031657
Nitric Oxide Signaling and Its Association with Ubiquitin-Mediated Proteasomal Degradation in Plants
Erratum in
-
Correction: Pande et al. Nitric Oxide Signaling and Its Association with Ubiquitin-Mediated Proteasomal Degradation in Plants. Int. J. Mol. Sci. 2022, 23, 1657.Int J Mol Sci. 2022 May 18;23(10):5628. doi: 10.3390/ijms23105628. Int J Mol Sci. 2022. PMID: 35628671 Free PMC article.
Abstract
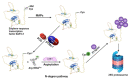
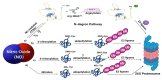
Nitric oxide (NO) is a versatile signaling molecule with diverse roles in plant biology. The NO-mediated signaling mechanism includes post-translational modifications (PTMs) of target proteins. There exists a close link between NO-mediated PTMs and the proteasomal degradation of proteins via ubiquitylation. In some cases, ubiquitin-mediated proteasomal degradation of target proteins is followed by an NO-mediated post-translational modification on them, while in other cases NO-mediated PTMs can regulate the ubiquitylation of the components of ubiquitin-mediated proteasomal machinery for promoting their activity. Another pathway that links NO signaling with the ubiquitin-mediated degradation of proteins is the N-degron pathway. Overall, these mechanisms reflect an important mechanism of NO signal perception and transduction that reflect a close association of NO signaling with proteasomal degradation via ubiquitylation. Therefore, this review provides insight into those pathways that link NO-PTMs with ubiquitylation.
Keywords: S-nitrosylation; nitric oxide; proteasome; proteolysis; ubiquitylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Acetylation modulates the Fanconi anemia pathway by protecting FAAP20 from ubiquitin-mediated proteasomal degradation.J Biol Chem. 2020 Oct 2;295(40):13887-13901. doi: 10.1074/jbc.RA120.015288. Epub 2020 Aug 6. J Biol Chem. 2020. PMID: 32763975 Free PMC article.
-
Plant Signaling: Ubiquitin Pulls the Trigger on Chloroplast Degradation.Curr Biol. 2016 Jan 11;26(1):R38-40. doi: 10.1016/j.cub.2015.11.022. Curr Biol. 2016. PMID: 26766233
-
Regulated proteolysis in light signaling.Curr Opin Plant Biol. 2005 Oct;8(5):469-76. doi: 10.1016/j.pbi.2005.07.002. Curr Opin Plant Biol. 2005. PMID: 16039154 Review.
-
Design Principles Involving Protein Disorder Facilitate Specific Substrate Selection and Degradation by the Ubiquitin-Proteasome System.J Biol Chem. 2016 Mar 25;291(13):6723-31. doi: 10.1074/jbc.R115.692665. Epub 2016 Feb 5. J Biol Chem. 2016. PMID: 26851277 Free PMC article. Review.
-
K63 ubiquitylation triggers proteasomal degradation by seeding branched ubiquitin chains.Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1401-E1408. doi: 10.1073/pnas.1716673115. Epub 2018 Jan 29. Proc Natl Acad Sci U S A. 2018. PMID: 29378950 Free PMC article.
Cited by
-
Genome-wide characterization of nitric oxide-induced NBS-LRR genes from Arabidopsis thaliana and their association in monocots and dicots.BMC Plant Biol. 2024 Oct 9;24(1):934. doi: 10.1186/s12870-024-05587-3. BMC Plant Biol. 2024. PMID: 39379841 Free PMC article.
-
Using brefeldin A to disrupt cell wall polysaccharide components in rice and nitric oxide to modify cell wall structure to change aluminum tolerance.Front Plant Sci. 2022 Aug 5;13:948212. doi: 10.3389/fpls.2022.948212. eCollection 2022. Front Plant Sci. 2022. PMID: 35991413 Free PMC article.
-
Hydrogen sulfide signaling in plant response to temperature stress.Front Plant Sci. 2024 Mar 13;15:1337250. doi: 10.3389/fpls.2024.1337250. eCollection 2024. Front Plant Sci. 2024. PMID: 38545385 Free PMC article. Review.
-
The Ubiquitin-26S Proteasome System-A Versatile Player Worthy of Close Attention in Plants.Int J Mol Sci. 2023 May 3;24(9):8185. doi: 10.3390/ijms24098185. Int J Mol Sci. 2023. PMID: 37175891 Free PMC article.
-
Nitric Oxide in Plant Functioning: Metabolism, Signaling, and Responses to Infestation with Ecdysozoa Parasites.Biology (Basel). 2023 Jun 28;12(7):927. doi: 10.3390/biology12070927. Biology (Basel). 2023. PMID: 37508359 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

