PaOctβ2R: Identification and Functional Characterization of an Octopamine Receptor Activating Adenylyl Cyclase Activity in the American Cockroach Periplaneta americana
- PMID: 35163598
- PMCID: PMC8835733
- DOI: 10.3390/ijms23031677
PaOctβ2R: Identification and Functional Characterization of an Octopamine Receptor Activating Adenylyl Cyclase Activity in the American Cockroach Periplaneta americana
Abstract
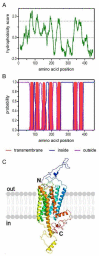
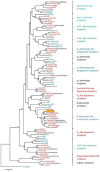
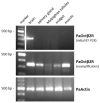
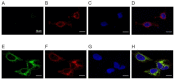
Biogenic amines constitute an important group of neuroactive substances that control and modulate various neural circuits. These small organic compounds engage members of the guanine nucleotide-binding protein coupled receptor (GPCR) superfamily to evoke specific cellular responses. In addition to dopamine- and 5-hydroxytryptamine (serotonin) receptors, arthropods express receptors that are activated exclusively by tyramine and octopamine. These phenolamines functionally substitute the noradrenergic system of vertebrates Octopamine receptors that are the focus of this study are classified as either α- or β-adrenergic-like. Knowledge on these receptors is scarce for the American cockroach (Periplaneta americana). So far, only an α-adrenergic-like octopamine receptor that primarily causes Ca2+ release from intracellular stores has been studied from the cockroach (PaOctα1R). Here we succeeded in cloning a gene from cockroach brain tissue that encodes a β-adrenergic-like receptor and leads to cAMP production upon activation. Notably, the receptor is 100-fold more selective for octopamine than for tyramine. A series of synthetic antagonists selectively block receptor activity with epinastine being the most potent. Bioinformatics allowed us to identify a total of 19 receptor sequences that build the framework of the biogenic amine receptor clade in the American cockroach. Phylogenetic analyses using these sequences and receptor sequences from model organisms showed that the newly cloned gene is an β2-adrenergic-like octopamine receptor. The functional characterization of PaOctβ2R and the bioinformatics data uncovered that the monoaminergic receptor family in the hemimetabolic P. americana is similarly complex as in holometabolic model insects like Drosophila melanogaster and the honeybee, Apis mellifera. Thus, investigating these receptors in detail may contribute to a better understanding of monoaminergic signaling in insect behavior and physiology.
Keywords: GPCR; biogenic amines; cellular signaling; cockroach; gene annotation; gene family; second messenger.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
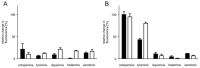
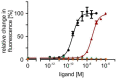
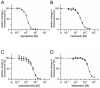
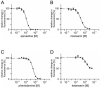
Similar articles
-
AmTAR2: Functional characterization of a honeybee tyramine receptor stimulating adenylyl cyclase activity.Insect Biochem Mol Biol. 2017 Jan;80:91-100. doi: 10.1016/j.ibmb.2016.12.004. Epub 2016 Dec 8. Insect Biochem Mol Biol. 2017. PMID: 27939988
-
PeaTAR1B: Characterization of a Second Type 1 Tyramine Receptor of the American Cockroach, Periplaneta americana.Int J Mol Sci. 2017 Oct 30;18(11):2279. doi: 10.3390/ijms18112279. Int J Mol Sci. 2017. PMID: 29084141 Free PMC article.
-
AmOctα2R: Functional Characterization of a Honeybee Octopamine Receptor Inhibiting Adenylyl Cyclase Activity.Int J Mol Sci. 2020 Dec 8;21(24):9334. doi: 10.3390/ijms21249334. Int J Mol Sci. 2020. PMID: 33302363 Free PMC article.
-
Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera.Arch Insect Biochem Physiol. 2001 Sep;48(1):13-38. doi: 10.1002/arch.1055. Arch Insect Biochem Physiol. 2001. PMID: 11519073 Review.
-
Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors.Invert Neurosci. 2005 Nov;5(3-4):111-8. doi: 10.1007/s10158-005-0001-z. Epub 2005 Oct 24. Invert Neurosci. 2005. PMID: 16211376 Review.
Cited by
-
Pharmacological Properties and Function of the PxOctβ3 Octopamine Receptor in Plutella xylostella (L.).Insects. 2022 Aug 16;13(8):735. doi: 10.3390/insects13080735. Insects. 2022. PMID: 36005359 Free PMC article.
-
Behavioral roles of biogenic amines in bumble bee males.Sci Rep. 2022 Dec 5;12(1):20946. doi: 10.1038/s41598-022-25656-7. Sci Rep. 2022. PMID: 36470960 Free PMC article.
-
Examination of Intracellular GPCR-Mediated Signaling with High Temporal Resolution.Int J Mol Sci. 2022 Jul 31;23(15):8516. doi: 10.3390/ijms23158516. Int J Mol Sci. 2022. PMID: 35955656 Free PMC article.
-
Molecular and Pharmacological Characterization of β-Adrenergic-like Octopamine Receptors in the Endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae).Int J Mol Sci. 2022 Nov 22;23(23):14513. doi: 10.3390/ijms232314513. Int J Mol Sci. 2022. PMID: 36498840 Free PMC article.
References
-
- Ohta H., Ozoe Y. Molecular signalling, pharmacology, and physiology of octopamine and tyramine receptors as potential insect pest control targets. Adv. Insect Physiol. 2014;46:73–166.
-
- Blenau W., Baumann A. Octopaminergic and tyraminergic signaling in the honeybee (Apis mellifera) brain: Behavioral, pharmacological, and molecular aspects. In: Farooqui A., editor. Trace Amines and Neurological Disorders. 1st ed. Academic Press; Oxford, UK: 2016. pp. 203–220.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

