Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress
- PMID: 35163602
- PMCID: PMC8835780
- DOI: 10.3390/ijms23031681
Functional Characterization of Tomato Phytochrome A and B1B2 Mutants in Response to Heat Stress
Abstract
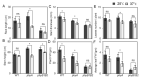
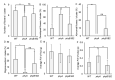
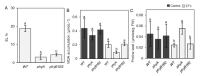
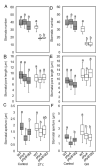
Heat stress (HS) is a prevalent negative factor affecting plant growth and development, as it is predominant worldwide and threatens agriculture on a large scale. PHYTOCHROMES (PHYs) are photoreceptors that control plant growth and development, and the stress signaling response partially interferes with their activity. PHYA, B1, and B2 are the most well-known PHY types in tomatoes. Our study aimed to identify the role of tomato 'Money Maker' phyA and phyB1B2 mutants in stable and fluctuating high temperatures at different growth stages. In the seed germination and vegetative growth stages, the phy mutants were HS tolerant, while during the flowering stage the phy mutants revealed two opposing roles depending on the HS exposure period. The response of the phy mutants to HS during the fruiting stage showed similarity to WT. The most obvious stage that demonstrated phy mutants' tolerance was the vegetative growth stage, in which a high degree of membrane stability and enhanced water preservation were achieved by the regulation of stomatal closure. In addition, both mutants upregulated the expression of heat-responsive genes related to heat tolerance. In addition to lower malondialdehyde accumulation, the phyA mutant enhanced proline levels. These results clarified the response of tomato phyA and phyB1B2 mutants to HS.
Keywords: HS; PHYTOCHROME A; PHYTOCHROME B1B2; heat tolerance; phyA; phyB1B2; tomato.
Conflict of interest statement
H.H. is employed by Kaneka Corporation. H.E. and K.M. received research support from Kaneka Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Stimulation of Tomato Drought Tolerance by PHYTOCHROME A and B1B2 Mutations.Int J Mol Sci. 2023 Jan 13;24(2):1560. doi: 10.3390/ijms24021560. Int J Mol Sci. 2023. PMID: 36675076 Free PMC article.
-
Overexpression of homologous phytochrome genes in tomato: exploring the limits in photoperception.J Exp Bot. 2007;58(3):615-26. doi: 10.1093/jxb/erl253. Epub 2007 Jan 23. J Exp Bot. 2007. PMID: 17251177
-
Tomato seed germination: regulation of different response modes by phytochrome B2 and phytochrome A.Plant Cell Environ. 2006 Apr;29(4):701-9. doi: 10.1111/j.1365-3040.2005.01455.x. Plant Cell Environ. 2006. PMID: 17080619
-
Shedding (far-red) light on phytochrome mechanisms and responses in land plants.Plant Sci. 2014 Mar;217-218:36-46. doi: 10.1016/j.plantsci.2013.11.013. Epub 2013 Nov 28. Plant Sci. 2014. PMID: 24467894 Review.
-
Photomorphogenic mutants of tomato.Biochem Soc Symp. 1994;60:249-56. Biochem Soc Symp. 1994. PMID: 7639784 Review. No abstract available.
Cited by
-
SlIAA9 Mutation Maintains Photosynthetic Capabilities under Heat-Stress Conditions.Plants (Basel). 2023 Jan 13;12(2):378. doi: 10.3390/plants12020378. Plants (Basel). 2023. PMID: 36679090 Free PMC article.
-
Photobiotechnology for abiotic stress resilient crops: Recent advances and prospects.Heliyon. 2023 Sep 15;9(9):e20158. doi: 10.1016/j.heliyon.2023.e20158. eCollection 2023 Sep. Heliyon. 2023. PMID: 37810087 Free PMC article. Review.
-
CRISPR/Cas9 Technique for Temperature, Drought, and Salinity Stress Responses.Curr Issues Mol Biol. 2022 Jun 8;44(6):2664-2682. doi: 10.3390/cimb44060182. Curr Issues Mol Biol. 2022. PMID: 35735623 Free PMC article. Review.
-
Application of Multi-Omics Technologies to the Study of Phytochromes in Plants.Antioxidants (Basel). 2024 Jan 14;13(1):99. doi: 10.3390/antiox13010099. Antioxidants (Basel). 2024. PMID: 38247523 Free PMC article. Review.
-
Engineering Abiotic Stress Tolerance in Crop Plants through CRISPR Genome Editing.Cells. 2022 Nov 13;11(22):3590. doi: 10.3390/cells11223590. Cells. 2022. PMID: 36429019 Free PMC article. Review.
References
-
- Wahid A., Gelani S., Ashraf M., Foolad M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. - DOI
-
- Silva R.S., Kumar L., Shabani F., Picanço M.C. Assessing the Impact of Global Warming on Worldwide Open Field Tomato Cultivation through CSIRO-Mk3·0 Global Climate Model. J. Agric. Sci. 2017;155:407–420. doi: 10.1017/S0021859616000654. - DOI
MeSH terms
Substances
Grants and funding
- 20K21302/Japan Society for the Promotion of Science
- (JST-OPERA, JPMJOP1851)/a Cooperative Research Grant of the Plant Transgenic Design Initiative (PTraD) by the Gene Re-search Center, Tsukuba-Plant Innovation Research Center (T-PIRC), and Program on Open In-novation Platform with Enterprise, Research Institute and Academia, Japa
LinkOut - more resources
Full Text Sources

