Remodeling of Cell Wall Components in Root Nodules and Flower Abscission Zone under Drought in Yellow Lupine
- PMID: 35163603
- PMCID: PMC8836056
- DOI: 10.3390/ijms23031680
Remodeling of Cell Wall Components in Root Nodules and Flower Abscission Zone under Drought in Yellow Lupine
Abstract
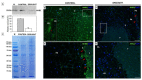
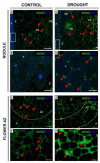
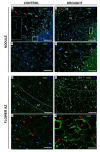
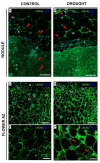
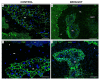
We recently showed that yellow lupine is highly sensitive to soil water deficits since this stressor disrupts nodule structure and functioning, and at the same time triggers flower separation through abscission zone (AZ) activation in the upper part of the plant. Both processes require specific transformations including cell wall remodeling. However, knowledge about the involvement of particular cell wall elements in nodulation and abscission in agronomically important, nitrogen-fixing crops, especially under stressful conditions, is still scarce. Here, we used immuno-fluorescence techniques to visualize dynamic changes in cell wall compounds taking place in the root nodules and flower AZ of Lupinus luteus following drought. The reaction of nodules and the flower AZ to drought includes the upregulation of extensins, galactans, arabinans, xylogalacturonan, and xyloglucans. Additionally, modifications in the localization of high- and low-methylated homogalacturonans and arabinogalactan proteins were detected in nodules. Collectively, we determined for the first time the drought-associated modification of cell wall components responsible for their remodeling in root nodules and the flower AZ of L. luteus. The involvement of these particular molecules and their possible interaction in response to stress is also deeply discussed herein.
Keywords: abscission zone; arabinan; cell wall; drought; extensins; galactans; root nodules; xyloglucans; yellow lupine; yielding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Analysis of Pectins, Extensins, and Hemicelluloses in Abscission Zones.Methods Mol Biol. 2025;2916:39-54. doi: 10.1007/978-1-0716-4470-6_4. Methods Mol Biol. 2025. PMID: 40366584
-
Drought Disrupts Auxin Localization in Abscission Zone and Modifies Cell Wall Structure Leading to Flower Separation in Yellow Lupine.Int J Mol Sci. 2020 Sep 18;21(18):6848. doi: 10.3390/ijms21186848. Int J Mol Sci. 2020. PMID: 32961941 Free PMC article.
-
De novo Transcriptome Profiling of Flowers, Flower Pedicels and Pods of Lupinus luteus (Yellow Lupine) Reveals Complex Expression Changes during Organ Abscission.Front Plant Sci. 2017 May 2;8:641. doi: 10.3389/fpls.2017.00641. eCollection 2017. Front Plant Sci. 2017. PMID: 28512462 Free PMC article.
-
IDA: a peptide ligand regulating cell separation processes in Arabidopsis.J Exp Bot. 2013 Dec;64(17):5253-61. doi: 10.1093/jxb/ert338. Epub 2013 Oct 22. J Exp Bot. 2013. PMID: 24151306 Review.
-
Genetic diversity of rhizobia associated with root nodules of white lupin (Lupinus albus L.) in Tunisian calcareous soils.Syst Appl Microbiol. 2019 Jul;42(4):448-456. doi: 10.1016/j.syapm.2019.04.002. Epub 2019 Apr 13. Syst Appl Microbiol. 2019. PMID: 31031015 Review.
Cited by
-
Genome-Wide Analysis of the Wall-Associated Kinase (WAK) Genes in Medicago truncatula and Functional Characterization of MtWAK24 in Response to Pathogen Infection.Plants (Basel). 2023 Apr 30;12(9):1849. doi: 10.3390/plants12091849. Plants (Basel). 2023. PMID: 37176907 Free PMC article.
-
Exploring the response of yellow lupine (Lupinus luteus L.) root to drought mediated by pathways related to phytohormones, lipid, and redox homeostasis.BMC Plant Biol. 2024 Nov 6;24(1):1049. doi: 10.1186/s12870-024-05748-4. BMC Plant Biol. 2024. PMID: 39506671 Free PMC article.
-
Analysis of Pectins, Extensins, and Hemicelluloses in Abscission Zones.Methods Mol Biol. 2025;2916:39-54. doi: 10.1007/978-1-0716-4470-6_4. Methods Mol Biol. 2025. PMID: 40366584
-
Transcriptome and targeted hormone metabolome reveal the molecular mechanisms of flower abscission in camellia.Front Plant Sci. 2022 Dec 22;13:1076037. doi: 10.3389/fpls.2022.1076037. eCollection 2022. Front Plant Sci. 2022. PMID: 36618654 Free PMC article.
-
Immunocytochemical Detection of Phytohormones and Phytohormone-Related Compounds in Abscission Zones.Methods Mol Biol. 2025;2916:1-15. doi: 10.1007/978-1-0716-4470-6_1. Methods Mol Biol. 2025. PMID: 40366581
References
-
- Fernández-Pascual M., Pueyo J.J., Felipe M., Golvano M.P., Lucas M.M. Singular features of the Bradyrhizobium-Lupinus symbiosis. Soil Dyn. Plant. 2007;1:1–16.
-
- Wilmowicz E., Kućko A., Golińska P., Burchardt S., Przywieczerski T., Świdziński M., Brzozowska P., Kapuścińska D. Abscisic acid and ethylene in the control of nodule-specific response on drought in yellow lupine. Environ. Exp. Bot. 2020;169:103900. doi: 10.1016/j.envexpbot.2019.103900. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

