Potential of Biofermentative Unsulfated Chondroitin and Hyaluronic Acid in Dermal Repair
- PMID: 35163608
- PMCID: PMC8835970
- DOI: 10.3390/ijms23031686
Potential of Biofermentative Unsulfated Chondroitin and Hyaluronic Acid in Dermal Repair
Abstract
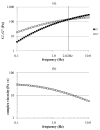
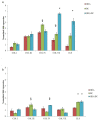
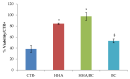
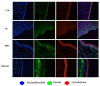
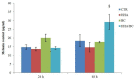
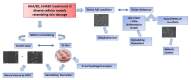
Chondroitin obtained through biotechnological processes (BC) shares similarities with both chondroitin sulfate (CS), due to the dimeric repetitive unit, and hyaluronic acid (HA), as it is unsulfated. In the framework of this experimental research, formulations containing BC with an average molecular size of about 35 KDa and high molecular weight HA (HHA) were characterized with respect to their rheological behavior, stability to enzymatic hydrolysis and they were evaluated in different skin damage models. The rheological characterization of the HHA/BC formulation revealed a G' of 92 ± 3 Pa and a G″ of 116 ± 5 Pa and supported an easy injectability even at a concentration of 40 mg/mL. HA/BC preserved the HHA fraction better than HHA alone. BTH was active on BC alone only at high concentration. Assays on scratched keratinocytes (HaCaT) monolayers showed that all the glycosaminoglycan formulations accelerated cell migration, with HA/BC fastening healing 2-fold compared to the control. In addition, in 2D HaCaT cultures, as well as in a 3D skin tissue model HHA/BC efficiently modulated mRNA and protein levels of different types of collagens and elastin remarking a functional tissue physiology. Finally, immortalized human fibroblasts were challenged with TNF-α to obtain an in vitro model of inflammation. Upon HHA/BC addition, secreted IL-6 level was lower and efficient ECM biosynthesis was re-established. Finally, co-cultures of HaCaT and melanocytes were established, showing the ability of HHA/BC to modulate melanin release, suggesting a possible effect of this specific formulation on the reduction of stretch marks. Overall, besides demonstrating the safety of BC, the present study highlights the potential beneficial effect of HHA/BC formulation in different damage dermal models.
Keywords: ECM biomarkers; chondroitin; hyaluronan gels; skin model; striae distensae; wound healing.
Conflict of interest statement
C.S., A.L.G. and A.D. are inventors but not assignee of the patent U.S. Patent Application No. 13/820,838; D.C. and C.S. are inventors but not assignee of the patent WO/2010/136435, International patent application PCT/EP2010/057129. There are no conflict of interest of the other co-author.
Figures








Similar articles
-
Q-switched Nd-YAG laser alone and in combination with innovative hyaluronic acid gels improve keratinocytes wound healing in vitro.Lasers Med Sci. 2021 Jul;36(5):1047-1057. doi: 10.1007/s10103-020-03145-5. Epub 2020 Sep 26. Lasers Med Sci. 2021. PMID: 32979135 Free PMC article.
-
In Vitro Evaluation of Novel Hybrid Cooperative Complexes in a Wound Healing Model: A Step Toward Improved Bioreparation.Int J Mol Sci. 2019 Sep 24;20(19):4727. doi: 10.3390/ijms20194727. Int J Mol Sci. 2019. PMID: 31554177 Free PMC article.
-
High molecular weight hyaluronic acid-liposome delivery system for efficient transdermal treatment of acute and chronic skin photodamage.Acta Biomater. 2024 Jul 1;182:171-187. doi: 10.1016/j.actbio.2024.05.026. Epub 2024 May 15. Acta Biomater. 2024. PMID: 38759743
-
Hyaluronan: More than just a wrinkle filler.Glycobiology. 2016 Jun;26(6):553-9. doi: 10.1093/glycob/cww033. Epub 2016 Mar 9. Glycobiology. 2016. PMID: 26964566 Free PMC article. Review.
-
Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects.Int J Biol Macromol. 2018 Dec;120(Pt B):1682-1695. doi: 10.1016/j.ijbiomac.2018.09.188. Epub 2018 Oct 1. Int J Biol Macromol. 2018. PMID: 30287361 Review.
Cited by
-
Effect of Marine-Derived Saccharides on Human Skin Fibroblasts and Dermal Papilla Cells.Mar Drugs. 2023 May 27;21(6):330. doi: 10.3390/md21060330. Mar Drugs. 2023. PMID: 37367655 Free PMC article.
-
Intradermal Injection of Hybrid Complexes of High- and Low-Molecular-Weight Hyaluronan: Where Do We Stand and Where Are We Headed in Regenerative Medicine?Int J Mol Sci. 2024 Mar 12;25(6):3216. doi: 10.3390/ijms25063216. Int J Mol Sci. 2024. PMID: 38542191 Free PMC article. Review.
-
Elastogenesis in Focus: Navigating Elastic Fibers Synthesis for Advanced Dermal Biomaterial Formulation.Adv Healthc Mater. 2024 Oct;13(27):e2400484. doi: 10.1002/adhm.202400484. Epub 2024 Jul 11. Adv Healthc Mater. 2024. PMID: 38989717 Free PMC article. Review.
-
Argan (Argania spinosa L.) Seed Oil Cake as a Potential Source of Protein-Based Film Matrix for Pharmaco-Cosmetic Applications.Int J Mol Sci. 2022 Jul 30;23(15):8478. doi: 10.3390/ijms23158478. Int J Mol Sci. 2022. PMID: 35955611 Free PMC article.
-
Protective and Anti-Inflammatory Effect of Novel Formulation Based on High and Low Molecular Weight Hyaluronic Acid and Salvia haenkei.Int J Mol Sci. 2025 Feb 4;26(3):1310. doi: 10.3390/ijms26031310. Int J Mol Sci. 2025. PMID: 39941078 Free PMC article.
References
-
- Plaas A.H., West L.A., Thonar E.J., Karcioglu Z.A., Smith C.J., Klintworth G.K., Hascall V.C. Altered Fine Structures of Corneal and Skeletal Keratan Sulfate and Chondroitin/Dermatan Sulfate in Macular Corneal Dystrophy. J. Biol. Chem. 2001;276:39788–39796. doi: 10.1074/jbc.M103227200. - DOI - PubMed
-
- Hochberg M.C., Altman R.D., April K.T., Benkhalti M., Guyatt G., McGowan J., Towheed T., Welch V., Wells G., Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–474. doi: 10.1002/acr.21596. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

