FAK in Cancer: From Mechanisms to Therapeutic Strategies
- PMID: 35163650
- PMCID: PMC8836199
- DOI: 10.3390/ijms23031726
FAK in Cancer: From Mechanisms to Therapeutic Strategies
Abstract
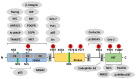
Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, is overexpressed and activated in many cancer types. FAK regulates diverse cellular processes, including growth factor signaling, cell cycle progression, cell survival, cell motility, angiogenesis, and the establishment of immunosuppressive tumor microenvironments through kinase-dependent and kinase-independent scaffolding functions in the cytoplasm and nucleus. Mounting evidence has indicated that targeting FAK, either alone or in combination with other agents, may represent a promising therapeutic strategy for various cancers. In this review, we summarize the mechanisms underlying FAK-mediated signaling networks during tumor development. We also summarize the recent progress of FAK-targeted small-molecule compounds for anticancer activity from preclinical and clinical evidence.
Keywords: combination therapy; drug resistance; focal adhesion kinase; metastasis; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

