3D Printed Graphene-PLA Scaffolds Promote Cell Alignment and Differentiation
- PMID: 35163657
- PMCID: PMC8836229
- DOI: 10.3390/ijms23031736
3D Printed Graphene-PLA Scaffolds Promote Cell Alignment and Differentiation
Abstract
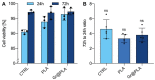
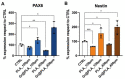
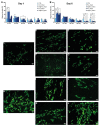
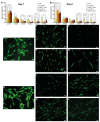
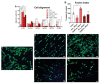
Traumas and chronic damages can hamper the regenerative power of nervous, muscle, and connective tissues. Tissue engineering approaches are promising therapeutic tools, aiming to develop reliable, reproducible, and economically affordable synthetic scaffolds which could provide sufficient biomimetic cues to promote the desired cell behaviour without triggering graft rejection and transplant failure. Here, we used 3D-printing to develop 3D-printed scaffolds based on either PLA or graphene@PLA with a defined pattern. Multiple regeneration strategies require a specific orientation of implanted and recruited cells to perform their function correctly. We tested our scaffolds with induced pluripotent stem cells (iPSC), neuronal-like cells, immortalised fibroblasts and myoblasts. Our results demonstrated that the specific "lines and ridges" 100 µm-scaffold topography is sufficient to promote myoblast and fibroblast cell alignment and orient neurites along with the scaffolds line pattern. Conversely, graphene is critical to promote cells differentiation, as seen by the iPSC commitment to neuroectoderm, and myoblast fusions into multinuclear myotubes achieved by the 100 µm scaffolds containing graphene. This work shows the development of a reliable and economical 3D-printed scaffold with the potential of being used in multiple tissue engineering applications and elucidates how scaffold micro-topography and graphene properties synergistically control cell differentiation.
Keywords: 3D printing; G+; Grafylon; PLA; graphene; neuronal differentiation; regenerative medicine; scaffold; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration.Acta Biomater. 2016 Dec;46:256-265. doi: 10.1016/j.actbio.2016.09.030. Epub 2016 Sep 22. Acta Biomater. 2016. PMID: 27667017
-
Development of new biocompatible 3D printed graphene oxide-based scaffolds.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110595. doi: 10.1016/j.msec.2019.110595. Epub 2019 Dec 24. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204059
-
Engineered 3D printed poly(ɛ-caprolactone)/graphene scaffolds for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Jul;100:759-770. doi: 10.1016/j.msec.2019.03.047. Epub 2019 Mar 16. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30948113
-
Electroactive Scaffolds for Neurogenesis and Myogenesis: Graphene-Based Nanomaterials.Small. 2018 Nov;14(48):e1801983. doi: 10.1002/smll.201801983. Epub 2018 Sep 27. Small. 2018. PMID: 30264534 Review.
-
Graphene-based 3D scaffolds in tissue engineering: fabrication, applications, and future scope in liver tissue engineering.Int J Nanomedicine. 2019 Jul 24;14:5753-5783. doi: 10.2147/IJN.S192779. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31413573 Free PMC article. Review.
Cited by
-
A Novel Graphene-Based Nanomaterial for the Development of a Pelvic Implant to Treat Pelvic Organ Prolapse.J Funct Biomater. 2024 Nov 20;15(11):351. doi: 10.3390/jfb15110351. J Funct Biomater. 2024. PMID: 39590554 Free PMC article.
-
Electric-Field-Driven Printed 3D Highly Ordered Microstructure with Cell Feature Size Promotes the Maturation of Engineered Cardiac Tissues.Adv Sci (Weinh). 2023 Apr;10(11):e2206264. doi: 10.1002/advs.202206264. Epub 2023 Feb 13. Adv Sci (Weinh). 2023. PMID: 36782337 Free PMC article.
-
Effect of the Interior Fill Percentage on the Deterioration of the Mechanical Properties of FFF-3D-Printed PLA Structures.Polymers (Basel). 2025 Mar 20;17(6):828. doi: 10.3390/polym17060828. Polymers (Basel). 2025. PMID: 40292715 Free PMC article.
-
The Mechanical, Thermal, and Chemical Properties of PLA-Mg Filaments Produced via a Colloidal Route for Fused-Filament Fabrication.Polymers (Basel). 2022 Dec 10;14(24):5414. doi: 10.3390/polym14245414. Polymers (Basel). 2022. PMID: 36559781 Free PMC article.
-
Skeletal Muscle Pathogenesis in Polyglutamine Diseases.Cells. 2022 Jul 3;11(13):2105. doi: 10.3390/cells11132105. Cells. 2022. PMID: 35805189 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

