A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells
- PMID: 35163665
- PMCID: PMC8836154
- DOI: 10.3390/ijms23031748
A Long Journey before Cycling: Regulation of Quiescence Exit in Adult Muscle Satellite Cells
Abstract
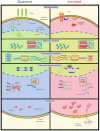
Skeletal muscle harbors a pool of stem cells called muscle satellite cells (MuSCs) that are mainly responsible for its robust regenerative capacities. Adult satellite cells are mitotically quiescent in uninjured muscles under homeostasis, but they exit quiescence upon injury to re-enter the cell cycle to proliferate. While most of the expanded satellites cells differentiate and fuse to form new myofibers, some undergo self-renewal to replenish the stem cell pool. Specifically, quiescence exit describes the initial transition of MuSCs from quiescence to the first cell cycle, which takes much longer than the time required for subsequent cell cycles and involves drastic changes in cell size, epigenetic and transcriptomic profiles, and metabolic status. It is, therefore, an essential period indispensable for the success of muscle regeneration. Diverse mechanisms exist in MuSCs to regulate quiescence exit. In this review, we summarize key events that occur during quiescence exit in MuSCs and discuss the molecular regulation of this process with an emphasis on multiple levels of intrinsic regulatory mechanisms. A comprehensive understanding of how quiescence exit is regulated will facilitate satellite cell-based muscle regenerative therapies and advance their applications in various disease and aging conditions.
Keywords: cell cycle re-entry; cell growth; checkpoints; mTORC1; quiescence exit; satellite cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

