Nanocarrier-Based Delivery of SN22 as a Tocopheryl Oxamate Prodrug Achieves Rapid Tumor Regression and Extends Survival in High-Risk Neuroblastoma Models
- PMID: 35163672
- PMCID: PMC8836113
- DOI: 10.3390/ijms23031752
Nanocarrier-Based Delivery of SN22 as a Tocopheryl Oxamate Prodrug Achieves Rapid Tumor Regression and Extends Survival in High-Risk Neuroblastoma Models
Abstract
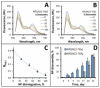
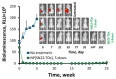
Despite the use of intensive multimodality therapy, the majority of high-risk neuroblastoma (NB) patients do not survive. Without significant improvements in delivery strategies, anticancer agents used as a first-line treatment for high-risk tumors often fail to provide clinically meaningful results in the settings of disseminated, recurrent, or refractory disease. By enhancing pharmacological selectivity, favorably shifting biodistribution, strengthening tumor cell killing potency, and overcoming drug resistance, nanocarrier-mediated delivery of topoisomerase I inhibitors of the camptothecin family has the potential to dramatically improve treatment efficacy and minimize side effects. In this study, a structurally enhanced camptothecin analog, SN22, reversibly coupled with a redox-silent tocol derivative (tocopheryl oxamate) to allow its optimally stable encapsulation and controlled release from PEGylated sub-100 nm nanoparticles (NP), exhibited strong NB cell growth inhibitory activity, translating into rapid regression and durably suppressed regrowth of orthotopic, MYCN-amplified NB tumors. The robust antitumor effects and markedly extended survival achieved in preclinical models recapitulating different phases of high-risk disease (at diagnosis vs. at relapse with an acquired loss of p53 function after intensive multiagent chemotherapy) demonstrate remarkable potential of SN22 delivered in the form of a hydrolytically cleavable superhydrophobic prodrug encapsulated in biodegradable nanocarriers as an experimental strategy for treating refractory solid tumors in high-risk cancer patients.
Keywords: SN22; bioluminescent imaging; drug resistance; high-risk disease; mitocan; nanoparticle; neuroblastoma; orthotopic xenograft model; prodrug; topoisomerase I inhibitor.
Conflict of interest statement
M. Chorny, G.M. Brodeur and I.S. Alferiev are inventors of a patent (US patent application number 20200061199). No potential conflict of interest is declared by the other authors.
Figures




References
-
- London W.B., Bagatell R., Weigel B.J., Fox E., Guo D., Van Ryn C., Naranjo A., Park J.R. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer. 2017;123:4914–4923. doi: 10.1002/cncr.30934. - DOI - PMC - PubMed
-
- Moreno L., Rubie H., Varo A., Le Deley M.C., Amoroso L., Chevance A., Garaventa A., Gambart M., Bautista F., Valteau-Couanet D., et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr. Blood Cancer. 2017;64:25–31. doi: 10.1002/pbc.26192. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

