Gene Transfer of Skeletal Muscle-Type Myosin Light Chain Kinase via Adeno-Associated Virus 6 Improves Muscle Functions in an Amyotrophic Lateral Sclerosis Mouse Model
- PMID: 35163674
- PMCID: PMC8836241
- DOI: 10.3390/ijms23031747
Gene Transfer of Skeletal Muscle-Type Myosin Light Chain Kinase via Adeno-Associated Virus 6 Improves Muscle Functions in an Amyotrophic Lateral Sclerosis Mouse Model
Abstract
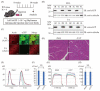
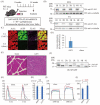
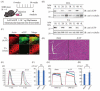
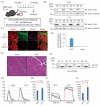
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that shows progressive muscle weakness. A few treatments exist including symptomatic therapies, which can prolong survival or reduce a symptom; however, no fundamental therapies have been found. As a therapeutic strategy, enhancing muscle force is important for patients' quality of life. In this study, we focused on skeletal muscle-specific myosin regulatory light chain kinase (skMLCK), which potentially enhances muscle contraction, as overexpression of skMLCK was thought to improve muscle function. The adeno-associated virus serotype 6 encoding skMLCK (AAV6/skMLCK) and eGFP (control) was produced and injected intramuscularly into the lower limbs of SOD1G37R mice, which are a familial ALS model. AAV6/skMLCK showed the successful expression of skMLCK in the muscle tissues. Although the control did not affect the muscle force in both of the WT and SOD1G37R mice, AAV6/skMLCK enhanced the twitch force of SOD1G37R mice and the tetanic force of WT and SOD1G37R mice. These results indicate that overexpression of skMLCK can enhance the tetanic force of healthy muscle as well as rescue weakened muscle function. In conclusion, the gene transfer of skMLCK has the potential to be a new therapy for ALS as well as for other neuromuscular diseases.
Keywords: adeno-associated virus 6; amyotrophic lateral sclerosis; gene therapy; muscle function; myosin light chain kinase.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Enhanced skeletal muscle contraction with myosin light chain phosphorylation by a calmodulin-sensing kinase.J Biol Chem. 2007 Jul 13;282(28):20447-54. doi: 10.1074/jbc.M702927200. Epub 2007 May 15. J Biol Chem. 2007. PMID: 17504755
-
Myosin light chain phosphorylation is required for peak power output of mouse fast skeletal muscle in vitro.Pflugers Arch. 2016 Nov;468(11-12):2007-2016. doi: 10.1007/s00424-016-1897-3. Epub 2016 Nov 28. Pflugers Arch. 2016. PMID: 27896430
-
The effect of skeletal myosin light chain kinase gene ablation on the fatigability of mouse fast muscle.J Muscle Res Cell Motil. 2011 Mar;31(5-6):337-48. doi: 10.1007/s10974-011-9239-8. Epub 2011 Feb 5. J Muscle Res Cell Motil. 2011. PMID: 21298329
-
Myosin phosphorylation improves contractile economy of mouse fast skeletal muscle during staircase potentiation.J Exp Biol. 2018 Jan 30;221(Pt 2):jeb167718. doi: 10.1242/jeb.167718. J Exp Biol. 2018. PMID: 29361581
-
Progressive impairment of CaV1.1 function in the skeletal muscle of mice expressing a mutant type 1 Cu/Zn superoxide dismutase (G93A) linked to amyotrophic lateral sclerosis.Skelet Muscle. 2016 Jun 23;6:24. doi: 10.1186/s13395-016-0094-6. eCollection 2016. Skelet Muscle. 2016. PMID: 27340545 Free PMC article.
Cited by
-
Skeletal muscle dysfunction in amyotrophic lateral sclerosis: a mitochondrial perspective and therapeutic approaches.Neurol Sci. 2024 Sep;45(9):4121-4131. doi: 10.1007/s10072-024-07508-6. Epub 2024 Apr 27. Neurol Sci. 2024. PMID: 38676818 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

