On-Growth and In-Growth Osseointegration Enhancement in PM Porous Ti-Scaffolds by Two Different Bioactivation Strategies: Alkali Thermochemical Treatment and RGD Peptide Coating
- PMID: 35163682
- PMCID: PMC8835960
- DOI: 10.3390/ijms23031750
On-Growth and In-Growth Osseointegration Enhancement in PM Porous Ti-Scaffolds by Two Different Bioactivation Strategies: Alkali Thermochemical Treatment and RGD Peptide Coating
Abstract
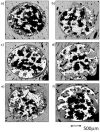
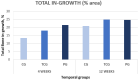
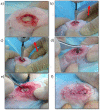
A lack of primary stability and osteointegration in metallic implants may result in implant loosening and failure. Adding porosity to metallic implants reduces the stress shielding effect and improves implant performance, allowing the surrounding bone tissue to grow into the scaffold. However, a bioactive surface is needed to stimulate implant osteointegration and improve mechanical stability. In this study, porous titanium implants were produced via powder sintering to create different porous diameters and open interconnectivity. Two strategies were used to generate a bioactive surface on the metallic foams: (1) an inorganic alkali thermochemical treatment, (2) grafting a cell adhesive tripeptide (RGD). RGD peptides exhibit an affinity for integrins expressed by osteoblasts, and have been reported to improve osteoblast adhesion, whereas the thermochemical treatment is known to improve titanium implant osseointegration upon implantation. Bioactivated scaffolds and control samples were implanted into the tibiae of rabbits to analyze the effect of these two strategies in vivo regarding bone tissue regeneration through interconnected porosity. Histomorphometric evaluation was performed at 4 and 12 weeks after implantation. Bone-to-implant contact (BIC) and bone in-growth and on-growth were evaluated in different regions of interest (ROIs) inside and outside the implant. The results of this study show that after a long-term postoperative period, the RGD-coated samples presented higher quantification values of quantified newly formed bone tissue in the implant's outer area. However, the total analyzed bone in-growth was observed to be slightly greater in the scaffolds treated with alkali thermochemical treatment. These results suggest that both strategies contribute to enhancing porous metallic implant stability and osteointegration, and a combination of both strategies might be worth pursuing.
Keywords: RGD peptide; bone in-growth; bone on-growth; histomorphometric evaluation; in vivo implantation; osseointegration; thermochemical treatment; titanium foams.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Two Different Strategies to Enhance Osseointegration in Porous Titanium: Inorganic Thermo-Chemical Treatment Versus Organic Coating by Peptide Adsorption.Int J Mol Sci. 2018 Aug 30;19(9):2574. doi: 10.3390/ijms19092574. Int J Mol Sci. 2018. PMID: 30200178 Free PMC article.
-
Novel porous Ti35Zr28Nb scaffolds fabricated by powder metallurgy with excellent osteointegration ability for bone-tissue engineering applications.Mater Sci Eng C Mater Biol Appl. 2019 Dec;105:110015. doi: 10.1016/j.msec.2019.110015. Epub 2019 Jul 26. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31546430
-
Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis.Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109908. doi: 10.1016/j.msec.2019.109908. Epub 2019 Jul 9. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31499974
-
Genetic potential of interfacial guided osteogenesis in implant devices.Dent Mater J. 2000 Jun;19(2):99-132. doi: 10.4012/dmj.19.99. Dent Mater J. 2000. PMID: 11219100 Review.
-
Porous construction and surface modification of titanium-based materials for osteogenesis: A review.Front Bioeng Biotechnol. 2022 Aug 25;10:973297. doi: 10.3389/fbioe.2022.973297. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36091459 Free PMC article. Review.
Cited by
-
Osseointegration of Implants Through Ti Biofunctionalization with Biomass from Chlorella sorokiniana UTEX 1230 and Synechococcus sp. PCC 7002.Int J Mol Sci. 2024 Dec 7;25(23):13161. doi: 10.3390/ijms252313161. Int J Mol Sci. 2024. PMID: 39684871 Free PMC article.
-
Biomimetic Coatings in Implant Dentistry: A Quick Update.J Funct Biomater. 2023 Dec 30;15(1):15. doi: 10.3390/jfb15010015. J Funct Biomater. 2023. PMID: 38248682 Free PMC article. Review.
-
Comparison of residual stress distribution between root-analogue implant and threaded cylindrical implant.BMC Oral Health. 2025 Jul 3;25(1):1080. doi: 10.1186/s12903-025-06412-5. BMC Oral Health. 2025. PMID: 40611171 Free PMC article.
-
Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review.Materials (Basel). 2024 Feb 5;17(3):769. doi: 10.3390/ma17030769. Materials (Basel). 2024. PMID: 38591985 Free PMC article. Review.
-
Hydrogel-Based Scaffolds: Advancing Bone Regeneration Through Tissue Engineering.Gels. 2025 Feb 27;11(3):175. doi: 10.3390/gels11030175. Gels. 2025. PMID: 40136878 Free PMC article. Review.
References
-
- Ahmadi S., Hedayati R., Li Y., Lietaert K., Tümer N., Fatemi A., Rans C., Pouran B., Weinans H., Zadpoor A., et al. Fatigue performance of additively manufactured meta-biomaterials: The effects of topology and material type. Acta Biomater. 2018;65:292–304. doi: 10.1016/j.actbio.2017.11.014. - DOI - PubMed
-
- Bobbert F., Lietaert K., Eftekhari A.A., Pouran B., Ahmadi S., Weinans H., Zadpoor A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017;53:572–584. doi: 10.1016/j.actbio.2017.02.024. - DOI - PubMed

