The Role of AGE-RAGE Signalling as a Modulator of Gut Permeability in Diabetes
- PMID: 35163688
- PMCID: PMC8836043
- DOI: 10.3390/ijms23031766
The Role of AGE-RAGE Signalling as a Modulator of Gut Permeability in Diabetes
Abstract
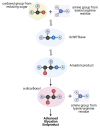
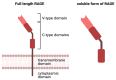
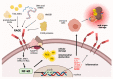
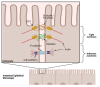
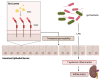
There is increasing evidence for the role of intestinal permeability as a contributing factor in the pathogenesis of diabetes; however, the molecular mechanisms are poorly understood. Advanced glycation endproducts, of both exogenous and endogenous origin, have been shown to play a role in diabetes pathophysiology, in part by their ligation to the receptor for advanced glycation endproducts (RAGE), leading to a proinflammatory signalling cascade. RAGE signalling has been demonstrated to play a role in the development of intestinal inflammation and permeability in Crohn's disease and ulcerative colitis. In this review, we explore the role of AGE-RAGE signalling and intestinal permeability and explore whether activation of RAGE on the intestinal epithelium may be a downstream event contributing to the pathogenesis of diabetes complications.
Keywords: advanced glycation endproducts; diabetes; intestinal permeability; receptor for advanced glycation endproducts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

