Mitochondrial Pathophysiology on Chronic Kidney Disease
- PMID: 35163697
- PMCID: PMC8836100
- DOI: 10.3390/ijms23031776
Mitochondrial Pathophysiology on Chronic Kidney Disease
Abstract
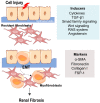
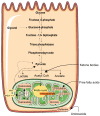
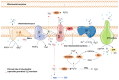
In healthy kidneys, interstitial fibroblasts are responsible for the maintenance of renal architecture. Progressive interstitial fibrosis is thought to be a common pathway for chronic kidney diseases (CKD). Diabetes is one of the boosters of CKD. There is no effective treatment to improve kidney function in CKD patients. The kidney is a highly demanding organ, rich in redox reactions occurring in mitochondria, making it particularly vulnerable to oxidative stress (OS). A dysregulation in OS leads to an impairment of the Electron transport chain (ETC). Gene deficiencies in the ETC are closely related to the development of kidney disease, providing evidence that mitochondria integrity is a key player in the early detection of CKD. The development of novel CKD therapies is needed since current methods of treatment are ineffective. Antioxidant targeted therapies and metabolic approaches revealed promising results to delay the progression of some markers associated with kidney disease. Herein, we discuss the role and possible origin of fibroblasts and the possible potentiators of CKD. We will focus on the important features of mitochondria in renal cell function and discuss their role in kidney disease progression. We also discuss the potential of antioxidants and pharmacologic agents to delay kidney disease progression.
Keywords: chronic kidney disease (CKD); electron transport phosphorylation (ETC) impairment; epithelial-mesenchymal transition (EMT); fibrosis; mitochondria; oxidative stress (OS).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential.Int J Mol Sci. 2021 Oct 19;22(20):11253. doi: 10.3390/ijms222011253. Int J Mol Sci. 2021. PMID: 34681922 Free PMC article. Review.
-
Mitochondrial dysfunction and the AKI-to-CKD transition.Am J Physiol Renal Physiol. 2020 Dec 1;319(6):F1105-F1116. doi: 10.1152/ajprenal.00285.2020. Epub 2020 Oct 19. Am J Physiol Renal Physiol. 2020. PMID: 33073587 Review.
-
Mitochondrial dysfunction: the hidden catalyst in chronic kidney disease progression.Ren Fail. 2025 Dec;47(1):2506812. doi: 10.1080/0886022X.2025.2506812. Epub 2025 May 29. Ren Fail. 2025. PMID: 40441691 Free PMC article. Review.
-
N-acetyl-cysteine increases cellular dysfunction in progressive chronic kidney damage after acute kidney injury by dampening endogenous antioxidant responses.Am J Physiol Renal Physiol. 2018 May 1;314(5):F956-F968. doi: 10.1152/ajprenal.00057.2017. Epub 2018 Jan 10. Am J Physiol Renal Physiol. 2018. PMID: 29357409 Free PMC article.
-
Single-cell transcriptomic profiling reveals decreased ER protein Reticulon3 drives the progression of renal fibrosis.Mol Biomed. 2024 Jun 28;5(1):24. doi: 10.1186/s43556-024-00187-x. Mol Biomed. 2024. PMID: 38937317 Free PMC article.
Cited by
-
Mitochondrial stress and glycoxidation increase with decreased kidney function.J Clin Biochem Nutr. 2023 Mar;72(2):147-156. doi: 10.3164/jcbn.22-101. Epub 2023 Mar 1. J Clin Biochem Nutr. 2023. PMID: 36936874 Free PMC article.
-
Serum sclerostin in vascular calcification in CKD: a meta-analysis.Ren Fail. 2023 Dec;45(1):2186151. doi: 10.1080/0886022X.2023.2186151. Ren Fail. 2023. PMID: 36880646 Free PMC article.
-
Protective Effects of Carnosol on Renal Interstitial Fibrosis in a Murine Model of Unilateral Ureteral Obstruction.Antioxidants (Basel). 2022 Nov 26;11(12):2341. doi: 10.3390/antiox11122341. Antioxidants (Basel). 2022. PMID: 36552549 Free PMC article.
-
A Cross-Sectional Study of Glomerular Hyperfiltration in Polycystic Ovary Syndrome.Int J Mol Sci. 2024 Apr 30;25(9):4899. doi: 10.3390/ijms25094899. Int J Mol Sci. 2024. PMID: 38732117 Free PMC article.
-
Genetic variants affecting mitochondrial function provide further insights for kidney disease.BMC Genomics. 2024 Jun 10;25(1):576. doi: 10.1186/s12864-024-10449-1. BMC Genomics. 2024. PMID: 38858654 Free PMC article.
References
-
- Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

